Introduction
Alaska pollock is a slender, snow-white fish with a delicate, flaky texture and a mild flavor that is caught in Alaska’s frigid, clean waters. To make surimi, the fish is combined with other ingredients and flavoring and shaped into a vast number of forms. Surimi is derived from fish mince that has been deboned and washed with water. This process results in a product that mostly consists of myofibrillar proteins and includes cryoprotectants. The washing procedure in frozen surimi manufacture is known to result in the removal of significant quantities of nutritious fish lipids. This technique is intended to enhance the concentration of myofibrillar proteins and increase the quality of frozen surimi (Jiao et al., 2019; Zhang et al., 2018). Lipids can hinder the gelation process of surimi-based products. For many years, experts and consumers have been interested in the health benefits of fish oil that contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
When people first tried to make kamaboko healthier by adding fat or oil to surimi, Ikeuchi & Simizu (1955) noticed that even a small amount of oil made the gel strength of surimi weaken. In a recent study, Shi et al. (2014) observed that different vegetable oils had a substantial impact on the ability of surimi gels to create a gel-like structure. Conversely, Okazaki et al. (2002, 2006) found that intense mixing reduced the size of oil particles and created a more stable emulsified surimi paste, leading to greater gel strength. In their study, Zhou et al. (2017) found that camellia tea oil could fill the vacant spaces within the surimi gel network, resulting in enhanced mechanical properties. Gao et al. (2018) and Niu et al. (2020) found that the addition of fish oil to surimi gels resulted in improvement when appropriate emulsification conditions were used.
The heating procedure is essential for achieving the required surimi gel with optimal elasticity, and varying heating techniques can result in different qualities of surimi products. Surimi goods are conventionally heated using a water bath. However, ohmic heating is commonly used as an alternative technique in the gelation process of surimi gel. Several studies have shown that ohmic heating can enhance the mechanical and functional characteristics of surimi gel (Fowler & Park, 2015; Nguyen et al., 2020; Tadpitchayangkoon et al., 2012; Yin & Park, 2015).
While some research has been done on emulsified gels, there aren’t any publications that look specifically at how marine-derived fish oil affects the quality and gelling properties of surimi gels that are heated sequentially using ohmic heating. This study was conducted to investigate the effects of adding fish oil to surimi paste on the gelling properties of Alaska pollock surimi gel. The study specifically focused on the potential degradation of the gel matrix when fish oil is added and how ohmic heating can improve the quality of the surimi gel. Moreover, the objective of this study is to determine the correlation between the emulsification levels of surimi enriched with fish oil and the circumstances of ohmic heating. This may enhance our understanding of the interaction between oil and protein during ohmic heating and offer novel approaches to creating fortified surimi-based goods using fish oil.
Materials and Methods
The Glacier Fish Company (Seattle, WA, USA) offered commercially frozen Alaska pollock (Theragra chalcogramma) surimi (RA grades) with 4% sucrose, 5% sorbitol, and 0.25% sodium polyphosphate. After delivery to the laboratory, a 10 kg block of surimi was cut into small pieces (~500 g), vacuum-wrapped, and stored in a freezer (–30°C) until needed.
The refined fish oil manufactured by Maruha Nichiro Corporation has a triglyceride content of above 99.9%, with DHA and EPA levels of 19.0% and 3.5%, respectively. The fish oil contained 34.7% saturated fatty acids and 65.3% unsaturated fatty acids. The fish oil was hermetically sealed with nitrogen gas (N2) and preserved in plastic bottles at a temperature of –20°C until it was ready to be utilized.
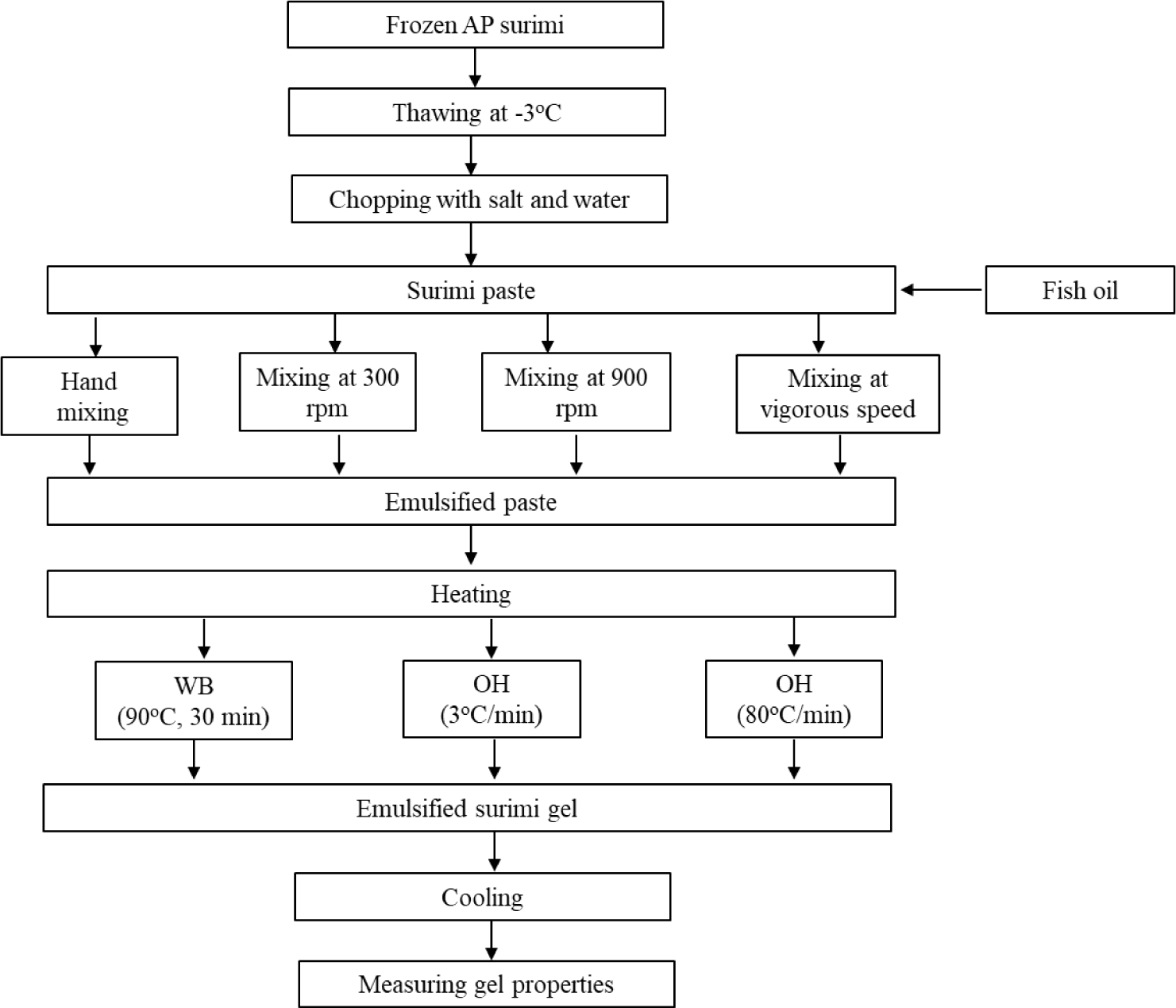
According to Okazaki et al. (2002), salted surimi paste was emulsified by varying the amount of fish oil used while keeping the protein-water ratio constant. To make the emulsified paste, frozen Alaska pollock surimi was thawed overnight at –3°C and chopped into small pieces (1–1.5 cm3). Surimi cubes were chopped at 1,500 rpm for 1.5 min under a 0.9 bar vacuum using a vacuum cutter (UMC-5, Stephan Machinery GmbH, Hameln, Germany) with a cooling system. The surimi paste was produced by mixing it with ice water and sodium chloride (1.5%, w/w). Table 1 displays the constituents of both the control and emulsified surimi paste. Following the addition of fish oil, a surimi paste that had been emulsified was made using four different mixing speeds: hand mixing, 300 rpm, 900 rpm, and vigorous mixing (Fig. 1). During manual mixing, surimi paste was blended with fish oil at a rotational speed of 300 revolutions per min for a duration of 8 min. A gradual rise in speed was used during high-speed mixing, starting at 300 rpm and ending at 3,000 rpm. The speed was increased in steps, with each step lasting for a specific duration. The first step was 300 rpm for 60 s, followed by 600, 900, 1,200, and 1,500 rpm for 30 s each. The next steps were 1,800 and 2,100 rpm for 15 s each, and finally 2,700 and 3,000 rpm for 10 s each. The experiment was replicated twice (Okazaki et al., 2002). The surimi paste was vacuum-sealed to eliminate any air pockets that may have formed during the chopping process.
| Surimi | IEW | Salt | Fish oil | Total | |
|---|---|---|---|---|---|
| Control | 600 | 188 | 12 | 0 | 800 |
| Emulsified surimi | 600 | 108 | 12 | 80 | 800 |
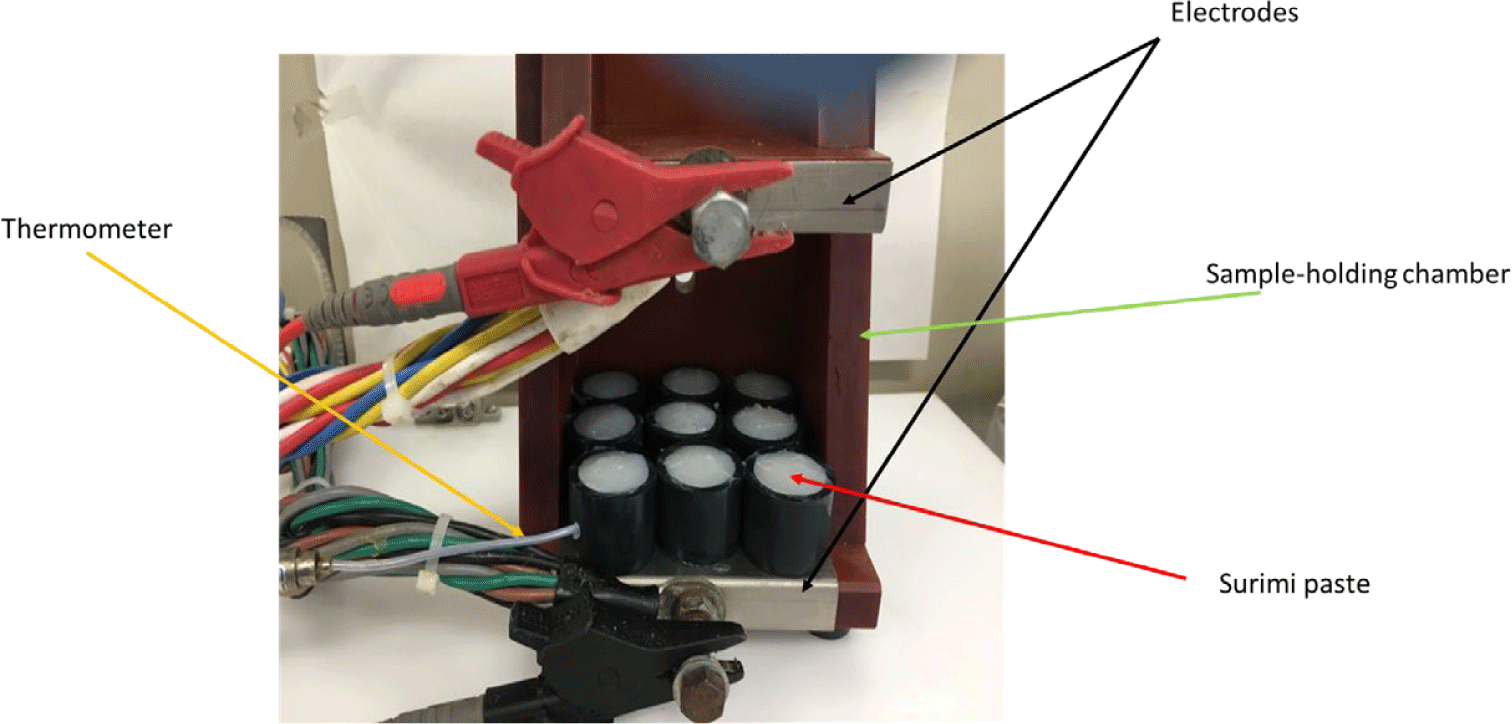
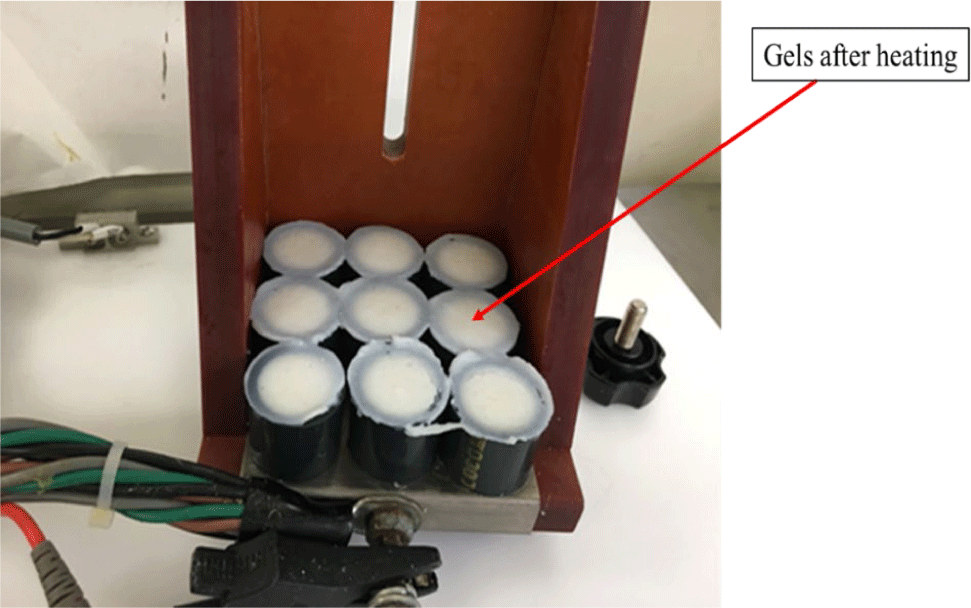
After chopping, a portion of the paste was inserted into plastic tubes with specific dimensions (inner diameter: 2.5 cm, length: 2.5 cm, tube thickness: 0.2 cm) and subsequently stored in a plastic bag. Next, the surimi paste was subjected to heat treatment by immersing it in a water bath set at a temperature of 90°C for a duration of 30 min. The excess was inserted into plastic tubes with a diameter of 2.5 cm and a length of 2.5 cm, and then subjected to ohmic heating (OH). 9 samples were positioned between two adjustable electrodes connected to the holding chamber. The upper electrode was thereafter forced downwards to create a secure connection with the tubes on both sides, ensuring a sample length of 2.5 cm. A thermocouple was used to monitor the temperature at the geometric center of the samples. The samples underwent heating from around 5°C to 90°C using two distinct voltage gradients that regulate the pace of heating: 3°C/min and 80°C/min. The heating conditions were depicted in the Table 2.
After the heat treatment, the samples were cooled by placing them in a plastic bag and immediately immersing them in ice or water at a temperature of 0°C–1°C for a duration of 30 min. Subsequently, the samples were stored in a refrigerator at a temperature of 4°C overnight, which lasted for 12–15 h (Nguyen et al., 2020). The process of thermally treating gels using ohmic heating was captured and depicted in Figs. 2 and 3.
Surimi paste was put into a polystyrene chamber between two titanium plate electrodes that faced each other at opposite ends of the chamber. The electrodes were 40 mm wide, 50 mm high, and 0.05 mm thick. This was done to test the electrical conductivity (EC) of the samples. The separation between the two electrodes was 30 mm. A consistent voltage of 50 volts was maintained across the electrodes until the desired temperature was achieved. According to Liu et al. (2007), the samples’ immediate Z, X, Rp, and Rs values (Z: impedance, C: capacitance, Rp: resistance parallel mode, Rs: resistance serries mode) were found using an liquidity coverage ratio (LCR) m (Hitester TESTER3532-50, HIOKI, Nagano, Japan) at a single point in time, when the temperature reached 5°C and the frequency was set to 20 kHz. The experiments were replicated five times. The EC, σ (S m−1), was determined based on electrode shape and resistance using the following equation:
where R is the electrical resistance of the food (Ω), L is the gap between the two electrodes (m), and A is the surface area of the electrodes in contact with surimi paste (m2).
A small amount of the emulsion was placed on a cooled slide glass (No. S2445, Matsunami glass, Bellingham, WA, USA) and the cover glass was spread thinly. The dispersion state of the fish oil was observed under fluorescence microscope BZ-9000E (Keyence, Osaka, Japan). The particle size distribution of the oil spheres on the microscopic image was measured and analyzed for fish oil particle diameter using image analysis software ImageJ developed by WayneRasband (ImageJ - http://imagej.nih.gov/ij/docs/index.html). The total area on the screen of particles of each size was calculated for each size, and the ratio to the area occupied by all oil balls was determined. The average particle size was determined to evaluate the effect of mixing conditions.
Breaking force (gf) (gel strength) and penetration distance (mm) (deformability) of surimi gels were determined using a texture analyzer (RE-3350B, Yamaden, Tokyo, Japan) as described by Rawdkuen et al. (2007). Six cylindrical samples (2.5 cm long) were prepared and equilibrated to room temperature (~25°C) for 1 h before analyses. A spherical probe (diameter 5 mm) penetrated into the center of the gel sample perpendicularly at a constant crosshead speed (60 mm/min). The force to puncture the gel (breaking force) and the distance from the point where the probe touched the gel to the point where the gel was punctured.
Expressible moisture was measured according to the method described by Rawdkuen & Benjakul (2008), with modifications. Gel samples were cut into thin slices (1.000 ± 0.025 g) (W1) and placed between 4 layers of filter paper (No. 4A paper on the inner side and No. 2 paper on the outer side). The filter papers were purchased from Advantec, Tokyo, Japan. Samples were then placed between 2 plastic plates (diameter: 4 cm) and a constant force (10 kg/cm2) was applied on top of the samples for 20 s using a rheometer (RE-3305, Yamaden, Tokyo, Japan) with 2 plastic plates attached to the crosshead (top) and the metal plate (bottom). Following this, the weight of the sample was measured again and recorded as W2. The expressible drip content was determined by applying the following equation and presenting the results as a percentage of the sample’s weight:
Samples were placed at room temperature (about 25°C) for 1 h prior to the color measurement. The color values of surimi gels samples were determined by Color Reader CR-13 (Konica Minolta, Marunouchi, Japan). Lightness (L*), redness (a*), and yellowness (b*) values were recorded respectively. The whiteness (W) was calculated using the equation:
Six replicates of measurements were taken.
Surimi pastes with varying emulsification levels, made using varied mixing speeds, were subjected to a dynamic rheological test. A rheometer (HAAKE MARS 60 Rheometer, Thermo Fisher Scientific, Yokohama, Japan) with a 35 mm parallel plate geometry was used to measure changes in storage (G′) or elastic modulus (G″). Once a linear response was observed in the viscoelastic area, an oscillation with a frequency of 1 Hz and a deformation of 1% was employed. The temperature sweep was recorded while the temperature increased from 5°C to 90°C using two distinct heating rates of 3 and 40°C/min. To reduce the evaporation of water from surimi pastes during measurement, a layer of paraffin oil was added to cover the samples (Gao et al., 2018).
Six samples were used for each measurement. The entire experiments were repeated twice. Mean values were subjected to analysis of variance (ANOVA) at a level of 95% significance using MS-Excel 2010 (Microsoft, Redmond, WA, USA). Error bars shown in figures indicate standard deviations of the values. Differences between the samples were compared statistically using Tukey’s method at a significant level of p < 0.05.
Results and Discussion
The EC of the research material is a vital attribute in ohmic heating technology. Table 3 exhibited a decline in the EC of emulsified surimi paste when fish oil was added. The mixing circumstances exerted a substantial influence on the EC, and the EC exhibited a positive association with the escalation in mixing velocity. EC is a complex connection between time and temperature, and it directly affects the rate of ohmic heating. Bozkurt & Icier (2010) state that the EC of food is influenced by not just the fat content, but also the size and distribution of the fat particles. The arrangement of fat globules in a meat product or mixture will impact the thickness of the samples and the growth in the size of the fat particles, resulting in a reduction in the movement of ions and consequently a decrease in EC (Okazaki et al., 2002). Changes in EC can lead to adjustments in heating conditions. To ensure the effective design of the ohmic heating system, it is crucial to gather and analyze data on the EC of foods, as well as their impact on key factors such as the frequency of the AC voltage, voltage gradient, and food matrix composition. Published data exists for high-frequency processes such as microwave, but it is not relevant for low-frequency ohmic heating processes. Only a limited number of articles (Fryer et al., 1993; Salengke & Sastry, 2007; Tulsiyan et al., 2008) have documented the electrical conductivities of actual food matrices, which consist of both liquid and solid components. It is commonly noted that the EC of liquids is greater than that of solids (Sastry & Palaniappan, 1992). The impact of the solids content in liquid foods is relatively less important but nonetheless noteworthy. Typically, when the proportion of solids increases (excluding salt), the EC decreases.
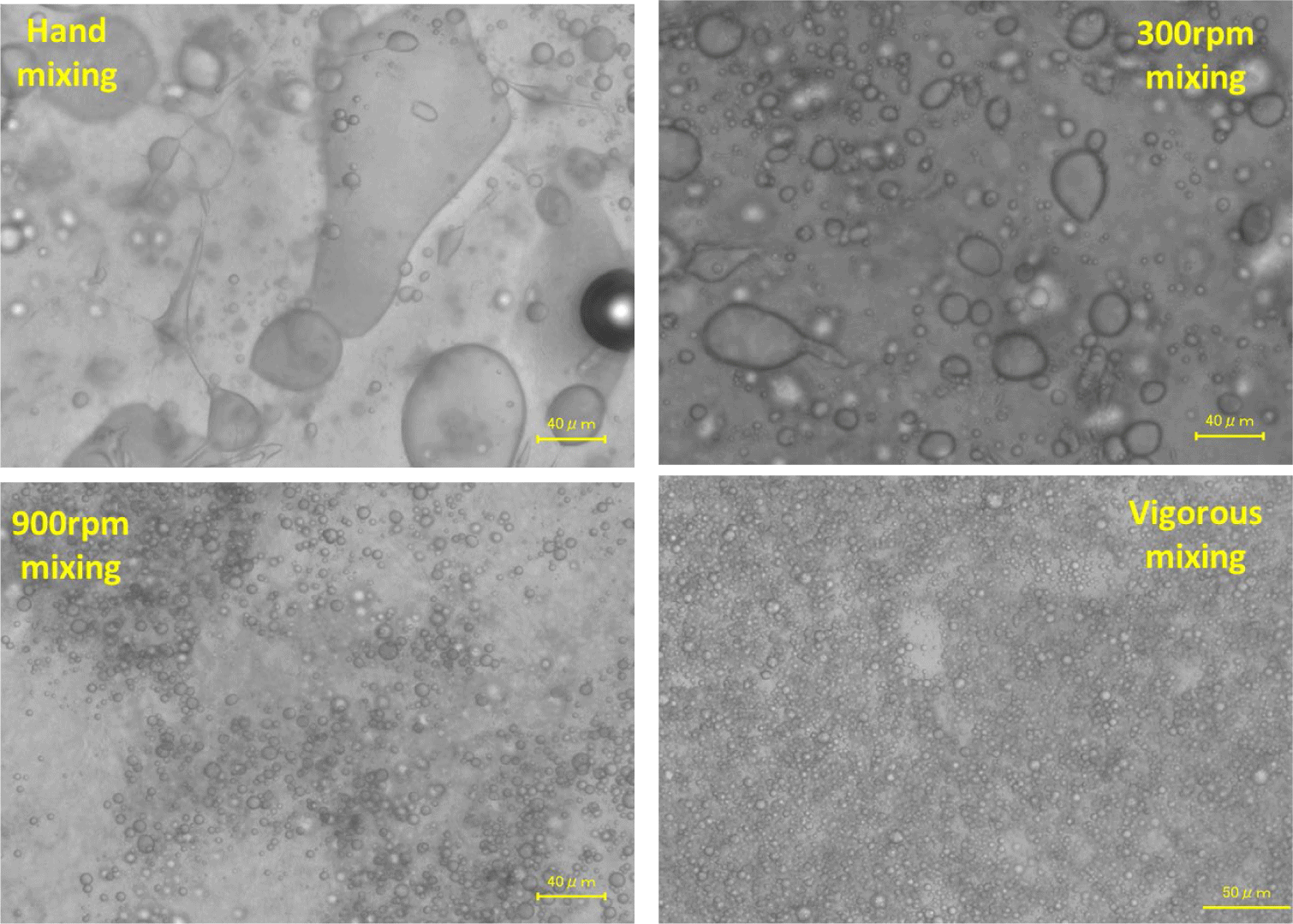
An investigation was conducted to examine the impact of mixing circumstances on the size of oil particles in surimi and fish oil. The microscopic observation photograph of several emulsions and the particle size distribution determined through image analysis are presented in Fig. 4 and Table 4, respectively. It was observed that the size of oil particles decreased as the mixing speed increased. Based on the microscopic examination depicted in the images of hand mixing and mixing at 300 rpm, numerous oil droplets of varying sizes and irregular shapes were seen. As the mixing speed increased, the oil particle size decreased dramatically, and the shape became more uniform. By employing intense agitation, the oil droplets achieved a size of approximately 3–5 μm. The oil particles were measured using ImageJ software, and the results were consistent with Table 4. The findings demonstrated that the velocity at which the components were mixed had a significant impact on the size of the droplets in microemulsions. Furthermore, high-speed mixing functioned as an emulsifying agent, hence enhancing the stability of the emulsion.
The impact of emulsifying fish oil on the breaking strength and breaking deformation of surimi gel generated by heating is illustrated in Fig. 5A and 5B. The emulsified level had a noticeable impact on the properties of the emulsified gel, with a statistically significant effect (p < 0.05). Insufficient emulsification of fish oil, either by hand or at a speed of 300 rpm, results in lower gelation ability compared to surimi without fish oil at the same protein concentration. The phrase “the product quality is reduced” is believed to pertain to the gel-forming capacity when fish oil is in a mixed state. However, if the emulsified state acquired with the gradual increase in speed during the awarding process, as used in this work, is attained, the inclusion of fish oil enhances the elasticity of the final product rather than diminishing it. Although the exact mechanism by which finely divided fish oil affects the ability of surimi to form a heated gel is not well understood, Jost et al. (1986) shown that it can decrease the size and uniformity of fat globules in whey protein gelation. Assuming that the creation of protein network structure is not obstructed, the increase in the ratio of protein adsorbed to the lipid-water interface, as the oil sphere surface area increases, leads to the immobilization of fat globules and the reinforcement of the gel structure. Thus, it appears that the gelation of surimi protein can be estimated in a similar manner.
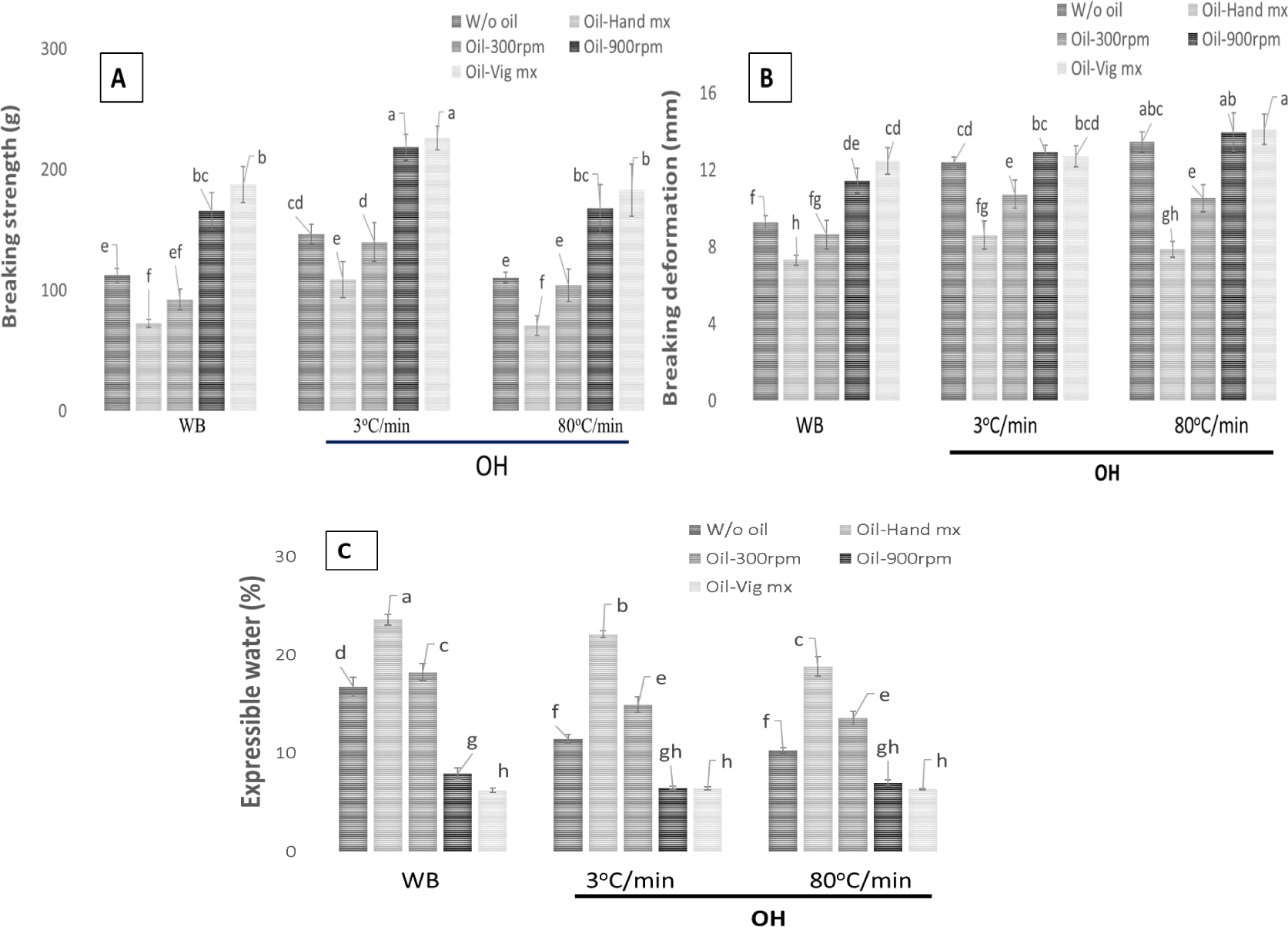
The gel characteristics of emulsified surimi paste were similarly affected by the heating rate caused by OH. Intense agitation reduced the number of oil droplets, created a consistent and stable emulsified surimi paste, and enhanced the gel strength throughout both water-bath heating and OH processing. The presence of a stable protein barrier surrounding the emulsified oil particle would enhance the stability of the gel structure and increase its breaking strength, as compared to a surimi gel that does not have additional fish oil.
Fig. 5C shows the measurable amount of liquid that is lost from a heated gel when it is subjected to a pressure of 10 kg for a duration of 20 s. The findings indicated a consistent decrease in the effective moisture content (EMC) of the gel formed by heat exposure as the amount of fish oil added increased. In general, when the EMC decreases, the water holding capacity (WHC) of surimi gels increases, leading to better gel strength and a more compact protein network. In their study, Fukushima et al. (2007) observed that the WHC of emulsified surimi gel improved with increasing amounts of fish oil. Additionally, the gel structure prevented oil droplets from being released when subjected to a compression force of 1 MPa. The addition of fish oil to the emulsified and heated gel results in a decrease in water content as the emulsification of fish oil increases. Specifically, the use of non-emulsified heated gel and 10% emulsified heated gel resulted in a drop of expressible drips from 6.5% to 4.0% when subjected to ohmic heating at a rate of 3°C/min. Uniform improvement in water retention was observed in all fish surimi, resulting in increased stability of the water content in the heated gel due to fish oil emulsification. However, we have shown that the gel strength decreases as the fish oil level increases (Fig. 5A), which is significant and goes against the previous thinking. There are two primary factors that can explain this phenomenon. The introduction of fish oil gradually decreases the moisture content in the test sample, leading to a reduced equilibrium moisture content in the gel with high oil content. Additionally, the presence of fish oil droplets plays a crucial role in preventing water migration within the gel network, thereby ensuring the stability of the oil-water-protein complex system (Liu et al., 2016). The ohmic heating gel has a reduced EMC compared to the WB group, which is strongly linked to the desirable features of the gel (Fig. 5). This conclusion aligns with the outcomes of several prior studies (Nguyen et al., 2020; Okazaki et al., 2002; Zhou et al., 2017).
The inclusion of fish oil resulted in a significant increase in the whiteness of all the gels, except for hand mixing, as seen in Table 5 (p < 0.05). The emulsified gel exhibited increased whiteness when made with greater mixing speeds (p < 0.05). The samples that were treated with fish oil and mixed vigorously exhibited the highest level of whiteness among all the samples (p < 0.05). The researchers found that the addition of soybean oil to mixed surimi made from bigeye snapper (SSA and SA grade) and mackerel resulted in an increase in whiteness, as demonstrated in the study conducted by Benjakul et al. in 2004. The enhanced whiteness of surimi when mixed with vegetable oil can be due to the phenomenon of light scattering caused by the emulsion formed when oil is combined with surimi and water (Park, 2000). Shi et al. (2014) found that the whiteness of silver carp surimi gel increased as the oil concentration rose. The addition of corn oil to Alaska pollock surimi gels resulted in increased whiteness, as seen in a study by Pietrowski et al. (2011). Manual mixing of the surimi paste resulted in poor mixing of the oil particles, leading to a reduction in the whiteness. The object had a golden or light-yellow color. By using fish oil and ensuring enough mixing speed, the little oil droplets were able to spread evenly throughout the gel matrix, resulting in enhanced light scattering. Hence, the inclusion of fish oil, particularly when vigorously mixed, directly enhanced the whiteness of the surimi gel.
There was no discernible disparity in the level of whiteness seen when comparing various heating rates or heating methods. Put simply, the choice of heating method (either water bath or ohmic heating) or heating rate (either 3°C/min or 80°C/min) did not have a noticeable impact on the whiteness of emulsified surimi gel (p < 0.05).
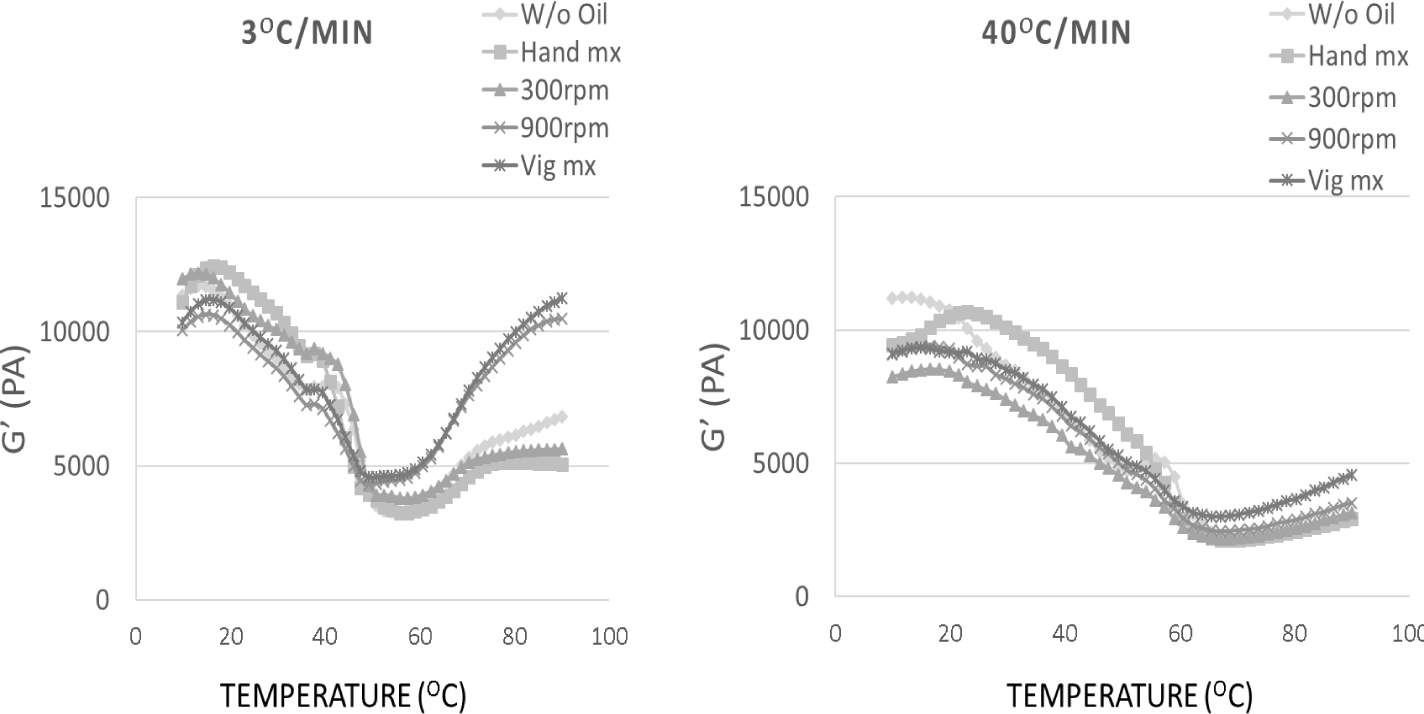
Fig. 6 illustrates the rheological characteristics of surimi paste with and without the addition of 10% fish oil, under various mixing speeds. The value of G′ gradually grew and reached its maximum at approximately 30°C. The initial rise in G′ at low temperatures may be attributed to the molecular interactions between actomyosin and the construction of a protein network structure by hydrogen bonding between protein molecules. This results in the formation of a less stable three-dimensional gel network (Zhang et al., 2013). Following that, the value of G′ declined at a temperature of 40°C and then sharply dropped to its lowest point at approximately 50°C for all the samples. At elevated temperatures, the protein aggregate formed was destabilized due to the destruction of a significant number of hydrogen bonds (Liu et al., 2007). In this temperature range, the gel structure can be broken down by the degradation of myosin, which is facilitated by endogenous proteolytic enzymes. This process is commonly referred to as ‘Modori’ (Gani & Benjakul, 2018). With continued heating, the value of G′ grew until it reached 65°C and then stayed nearly constant up to 90°C for the control sample, hand mixing, and mixing at 300 rpm. In the cases where the remaining speed was 900 rpm and the mixing was vigorous, the value of G′ consistently grew until the temperature hit 90°C. This value was notably greater than the values observed in the previously described cases. The occurrence could be attributed to the development of a stable gel structure linked to increased protein aggregation (Liu et al., 2007). There was a strong correlation between the increasing G′ values and the breaking strength values of the samples. The evolution and trends of the G′ as a function of heating temperature exhibited consistent behavior across all samples. In general, the G′ value was greater in the emulsified sample that was mixed at high rates, suggesting the development of a more solid gel network. Disrupting the muscle protein filaments can occur when mixing speeds are varied, such as with hand mixing or slow mixing, which can hinder the entanglement process during gelation. This was demonstrated by the decrease in the value of G when the mixing speed was slow. This outcome aligned with the findings on the strength and deformation at the point of failure.
Conclusions
The incorporation of fish oil with surimi enhanced the gel characteristics during both water-bath heating and ohmic heating processes. Vigorous mixing at high speeds resulted in a reduction in the size of oil droplets, leading to the formation of a homogenous and stable emulsified surimi paste. Additionally, this process contributed to an increase in the gel strength. The gel characteristics and gel-forming ability, as well as the EMC, of emulsified surimi paste were dramatically enhanced by the heating rate. The emulsified gels had the highest breaking strength and lowest drip loss when subjected to a moderate heating rate of 3°C/min, as compared to the other conditions (p < 0.05). The rheological and color measurements exhibited consistent findings. The findings demonstrated that a slow heating rate might be used to produce surimi-based goods enriched with fish oil by vigorous mixing. Implications of these results for the manufacture of superior surimi-based products are potentially critical.