Introduction
The climbing perch, Anabas testudineus (Bloch), locally called “Koi” is an Anabantoids fish widely distributed in Southeast Asia. Anabantoids have a lung-like organ called the labyrinth, which enables them to breathe oxygen directly from the air instead of depending on branchial respiration (Pinter, 1986). It mainly inhabits sluggish, standing, and or even stagnant water bodies such as swamps, canals, pools, small pits, and puddles in Bangladesh, India, Pakistan, Nepal, China, Myanmar, Thailand, Cambodia, Philippines, Indonesia, Singapore, and Sri Lanka (Hossain et al., 2015; Mawa et al., 2021). A. testudineus is an important commercial fish in Southeast Asia and is usually sold alive at a high price in local markets. There are recognizable differences in color, taste, and growth rate among different strains of climbing perch found in different countries. Climbing perch found in Bangladesh are smaller in size, dark grayish, and highly tolerant to adverse environments. The growth rate of native climbing perch koi is slow, taking more time to attain marketable size (Suraiya et al., 2012). Moreover, the fry collection of local climbing perch is difficult (Hossain et al., 2012).
In 2002, Thai A. testudineus was introduced in Bangladesh for cultural purposes. Although, the Thai strains have a high growth rate but lack natural odor and taste (Suraiya et al., 2012). In addition, due to the failure to maintain proper hatchery management activities including supplying feed, sampling ethics in the fry production phase, and inbreeding, resulted in the reduction of high-yielding characteristics of Thai A. testudineus (Habib et al., 2015) in Bangladesh. Vietnam-originated A. testudineus was introduced in Bangladesh by Sharnalata Agro Fisheries Ltd to overcome this problem, in 2011(Habib et al., 2015). According to the claim of the hatchery mentioned above, these Vietnam-originated breeds are growing almost four times faster than the conventional Thai variety. Moreover, the color of this Vietnam-originated perch resembles the color of its native counterpart which made them very lucrative to the consumers. The market for this strain as an aquaculture species grew swiftly, and commercial farming also gently increased. However, as Bangladesh is a flood-prone country, the chances of farmed Vietnam-originated climbing perch escaping in nature are very high. Its likelihood of escaping and possible crossbreeding with the native strain may cause genetic introgression and consequently morphological changes resulting in adaptation threat of native strain in nature. The comparative morphological variation is still lacking of these two strains and need to be documented.
Morphological analysis is considered a simple, cost-effective, and common tool to identify and characterize fish stocks (Siddik et al., 2016) and distinguish between fish populations (Siddik et al., 2015). Samoilov & Dien (2022) reported the morphological characterization of A. testudineus in Vietnam. In Bangladesh, few reports on the morpho-meristic variation of A. testudineus of the native and Thai strains have been delivered (Ara & Nabi, 2018; Hossen et al., 2017). Besides, no information is available regarding morphological characters and meristic count differences between native Bangladeshi and Vietnam-originated climbing perch in Bangladesh. Though, genetic variations of Bangladeshi and Vietnam-originated A. testudineus have been reported (Parvez et al., 2020). Thus, in the present study, native and introduced Vietnam-originated populations of climbing perch (A. testudineus) were collected, across Bangladesh, and performed morphological analyses and documentation for future reference.
Materials and Method
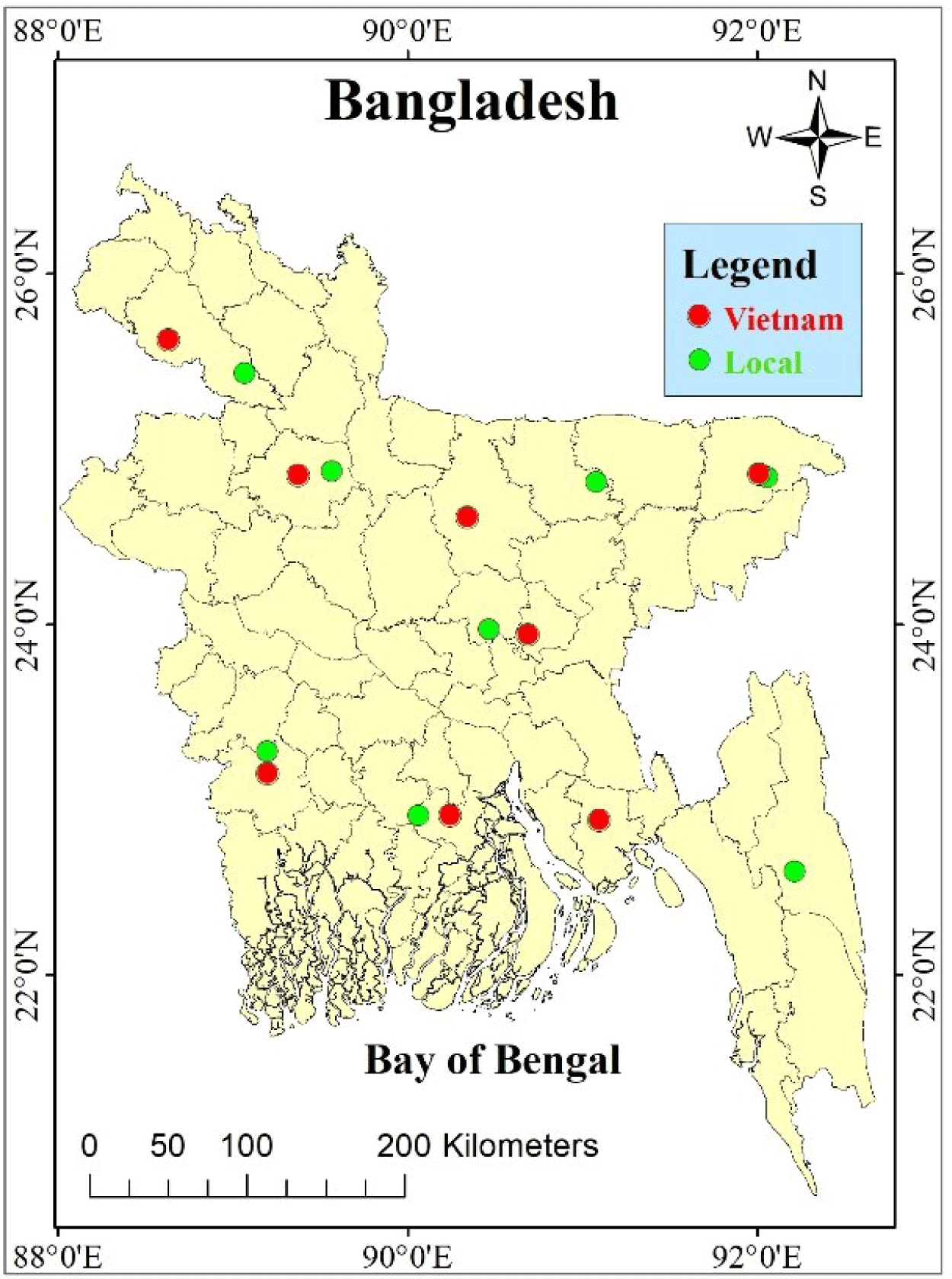
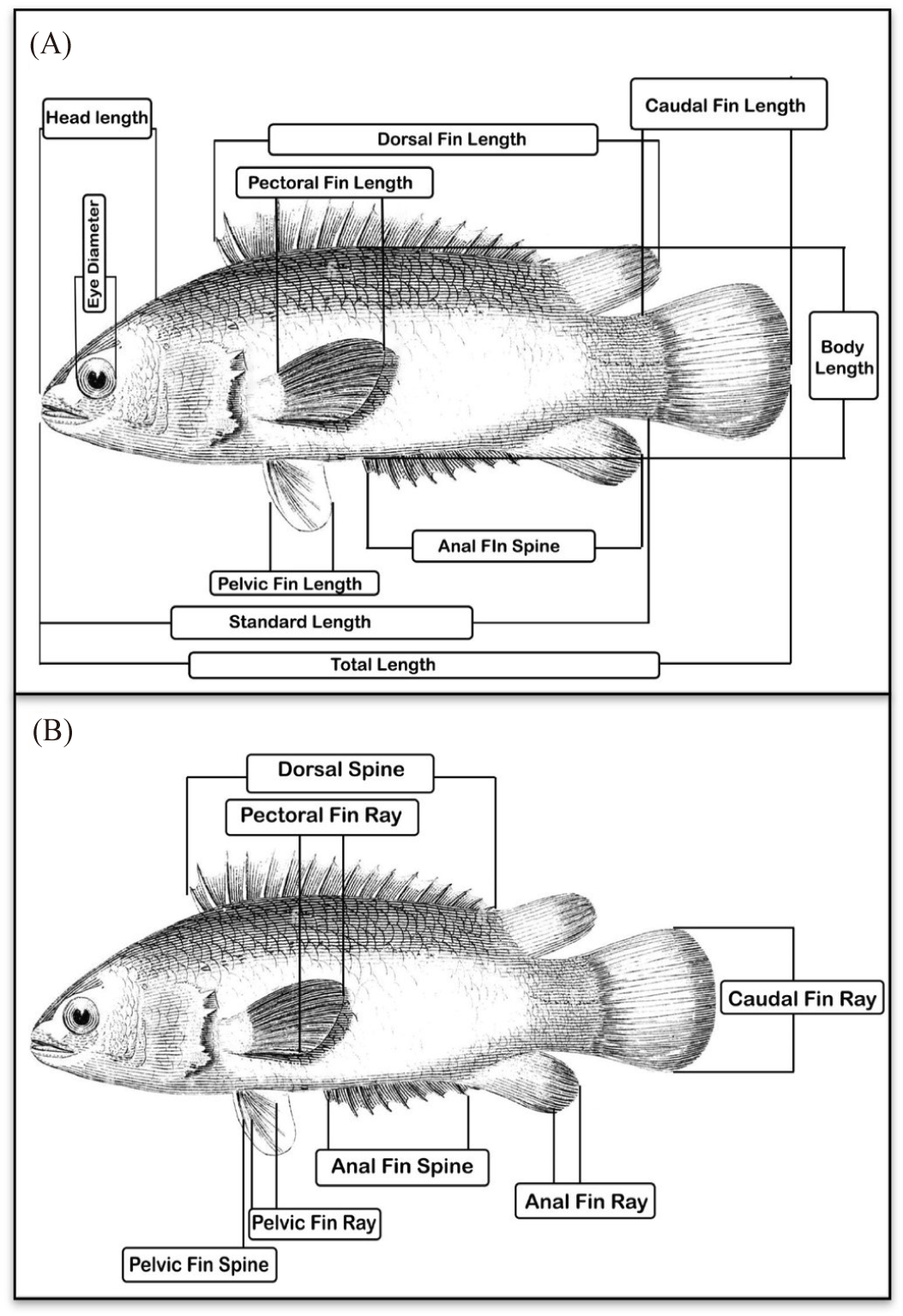
Experimental samples of climbing perch (A. testudineus) were collected from the natural habitat and cultural conditions of eight divisions of Bangladesh. The sixteen sampling stations are shown in Fig. 1. Detailed information on samples used in this research is given in Table 1. A total of 320 samples were used for morphometric and meristic analysis. Ten morphometric traits such as total length (TL), standard length (SL), head length (HL), body depth (BD), eye diameter (ED), dorsal fin length (DFL), pectoral fin length (PCFL), pelvic fin length (PVFL), anal fin length (AFL), caudal fin length (CFL) (Fig. 2A) and seven meristic counts such as pectoral fine ray (PCFR), pelvic fine ray (PVFR), caudal fin ray (CFR), dorsal fin spine (DFS), anal fin spine (AFS), pelvic fin spine (PVFS), and lateral line scale (LLS) (Fig. 2B) were recorded following our previous work (Rakhi et al., 2024).
Multivariate approaches were used to examine data from 10 morphometric and 7 meristic assessments of the native and Vietnam-originated individuals. Analysis was carried out separately for morphometric and meristic characters. Since meristic characters were independent of the size of the fish (Hossen, 2017; Strauss, 1985) the raw meristic data was used in the analysis. However, to avoid possible biases produced by size effects on the morphometric variables, all morphometric characters were standardized by the formula,
where,
ACi is the adjusted logarithmic character measurements of the ith specimen (i = 1, 2, 3...);
OCi is the unadjusted character measurement of the ith specimen (i = 1, 2, 3...);
β is the common within-group regression coefficient of that character against total length after the logarithmic transformation of both variables;
TLi is the total length of the ith specimen (i = 1, 2, 3...); and
MTL is the overall mean total length.
Through the use of the correlation between total length and adjusted characters, the effectiveness of the allometric formula in eliminating the size effect from the data was demonstrated. Therefore, total length was removed as a parameter, and other parameters were standardized using total length as a reference, following the methodology of Hossen et al. (2017).
Kruskal–Wallis test was used to compare means between the groups. The principal component analysis was conducted to identify the origins of the greatest amount of variance in the variables by assisting in data reduction. Cluster analysis using the ward linkage method was done separately for morphometric and meristic data to show the clustering pattern of these two strains. The data were statistically analyzed using various statistical software such as IBM SPSS, version 26.0. IBM Corp (Armonk, NY, USA) was used for the MANOVA test, and the Kruskal–Wallis (H) test. R tool (version 4.3.1) was used to perform the principal component analysis (PCA). A 5% level of significance threshold was used for all tests.
Results and Discussion
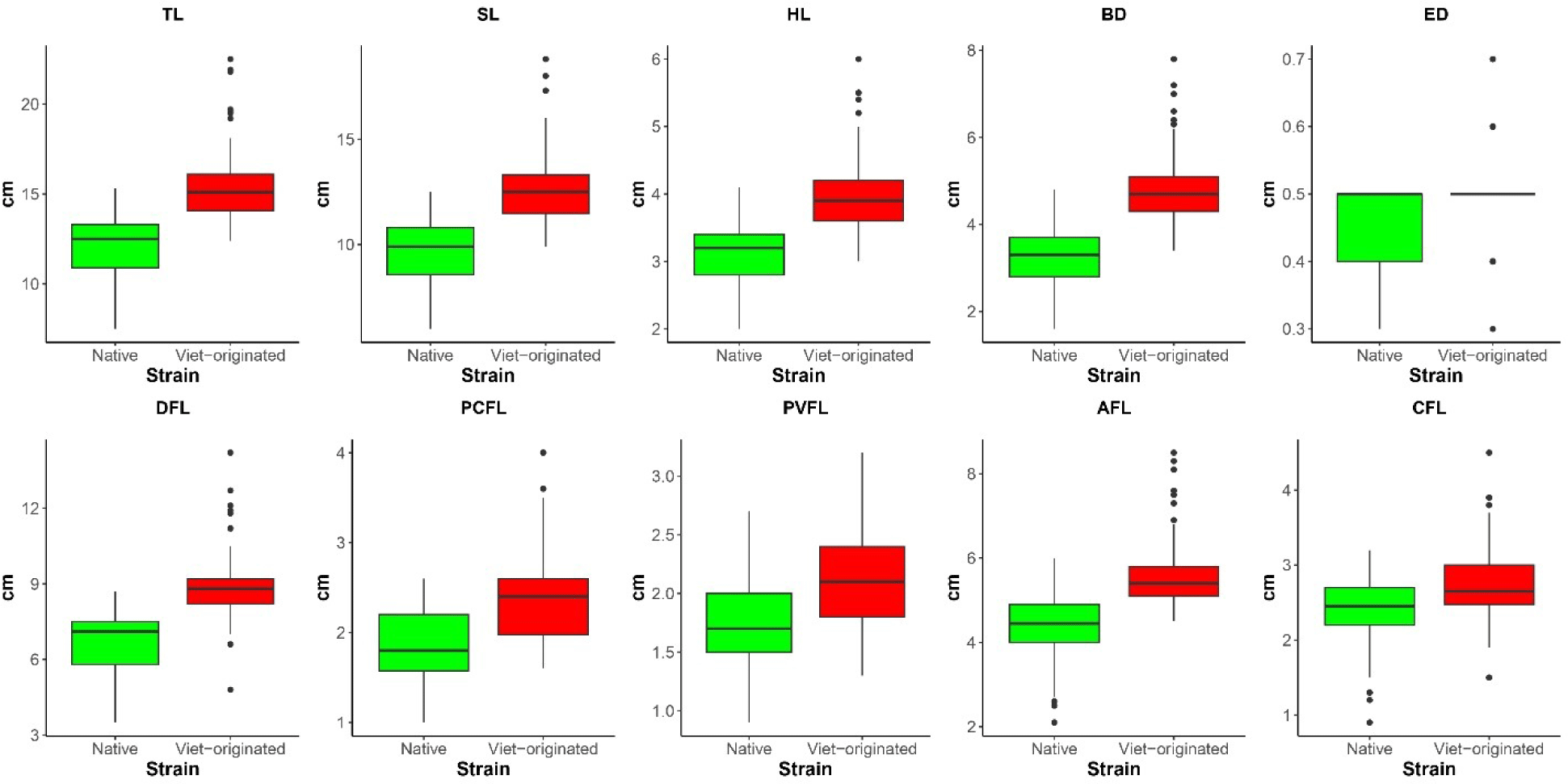
The native and Vietnam-originated strains collected from different locations showed a wide range of size variations and significant (p < 0.05) differences based on their morphometric characteristics and meristic counts. Spatial variation was also observed in both native and Vietnam-originated populations separately as they were collected from several natural sources and cultivated farms situated in different areas of Bangladesh. Table 2 shows the descriptive statistics and the coefficient of variation (CV%) of the 10 morphometric measures of the native and Vietnam-originated strains. The low CV% of each measurement indicated less variation and phenotypic homogeneity among the strains. The mean TL and SL of native strains were found 12.09 and 9.69 cm, whereas Vietnam-originated mean TL and SL were observed 13.68 and 11.12 cm, respectively. All other morphometric values were observed higher in Vietnam-originated A. testudineus. Among the morphometric characteristics TL, SL, HL, BD, ED, and PCFL were found to be very similar to the observation of Hassan et al. (2005), however, DFL slightly differed. A study by Ara & Nabi (2018) revealed that TL, SL, BD, and height of pectoral and pelvic fin length of Thai climbing perch were greater than native Bangladeshi strain. However, the head length, eye diameter length, postorbital length, predorsal length, and caudal peduncle length were similar. In terms of meristic counts, the number of DFS, AFS, and LLS were found to be different between the two strains whereas both the strains of climbing perch showed the same number of PVFS, CFR, PVFR, and PCFR (Table 3). The native strain had the maximum number (3.33%) of the dorsal fin spine. Around 51.25% of native samples and 76.25% of Vietnam-originated strains samples had 17 dorsal fin spines. The highest percentage (6.25%) of maximum anal fin spine count was observed in the native population. For native strains, the most common (67.5%) anal fin spine count observed was 10, and for Vietnam-originated strains (61.88%) it was 9. Similar to the anal fin spine, the highest percentage (3.12%) of maximum caudal fin ray count was observed in the native population. The most common caudal fin ray count in both native (59.38%) and Vietnam-originated strains (54.37%) observed is 16. The highest percentage (1.88%) of maximum pectoral fin ray count was observed in the native strains. The percentage of fish with the most common pectoral fin ray counts 14 in native is 58.13% and, in the Vietnam-originated, 13 is 36.25%. The highest percentage (3.75%) of maximum lateral line scale count was observed in the Vietnam-originated strain. The most common lateral line scale for both native (40.62%) and Vietnam-originated (31.25%) strains is 27. The meristic count of native A. testudineus obtained in this study was similar to Ara & Nabi (2018) except for the number of DFS (15–18), and LLS (30–38) as they found whereas it was 14–18 and 24–29 in the present study. The box whisker plot of the morphometric features demonstrated differences between the native and Vietnam-originated populations of A. testudineus. The morphological measurement showed highest ranges in the Vietnam-originated strains were greater than those of natives (Fig. 3). Outliers in native populations contributed significantly to data variation.
| Characteristics | Range | |
|---|---|---|
| Native | Vietnam-originated | |
| DFS | 14–18 | 15–18 |
| AFS | 9–11 | 8–11 |
| PVFS | 1 | 1 |
| CFR | 14–18 | 14–18 |
| PVFR | 5 | 5 |
| PCFR | 13–16 | 13–16 |
| LLS | 24–29 | 24–30 |
Multivariate analysis of variance (MANOVA) of morphometric data revealed significant variation (Wilk’s Lambda = 0.421, p < 0.05) in the case of SL, HL, BD, ED, DFL, PCFL, PVFL, and AFL between native and Vietnam-originated strains (Table 4). Kruskal-Wallis (H) test of the morphometric data showed that native and Vietnam-originated A. testudineus are significantly different from each other (p < 0.05) concerning SL, HL, BD, ED, DFL, PCFL, PVFL, AFL, and CFL (Table 4). The MANOVA of meristic characters revealed significant variation (Wilk’s Lambda = 0.734, p < 0.05) in the case of DFS, AFS, CFR, PCFR, and LLS (Table 4). Kruskal-Wallis (H) test of the meristic data showed that the two strains are significantly different from each other (p < 0.05) concerning DFS, AFS, CFR, PCFR, and LLS (Table 4). A study conducted by Hossen et al. (2017), reported significant variation (p < 0.01) between SL, BD, ED, DFL, and AFL but insignificant differences in the lowest body depth, PCFL, and PVFL of indigenous and climbing perch. However, the Kruskal-Wallis (H) test of morphometric characters between indigenous and Thai strains revealed a significant difference (p < 0.01) concerning SL, HL, BD, ED, LDF, PCVL, and AFL but an insignificant difference (p > 0.01) in PVFL. The study also revealed significant variation (p < 0.01) in the MANOVA test of DFS, DFR, PVFR, and AFS. Moreover, the Kruskal-Wallis (H) test showed a significant difference (p < 0.05) between native and Thai climbing perch based on DFS, DFR, PVFR, and AFS but an insignificant difference (p > 0.05) in PCFR, AFR, CFR.
SL, standard length; HL, head length; BD, body depth; ED, eye diameter; DFL, dorsal fin length; PCFL, pectoral fin length; PVFL, pelvic fin length; AFL, anal fin length; CFL, caudal fin length; DFS, dorsal fin spine; AFS, anal fin spine; PVFS, pelvic fin spine; CFR, caudal fin ray; PVFR, pelvic fine ray; PCFR, pectoral fine ray; LLS, lateral line scale.
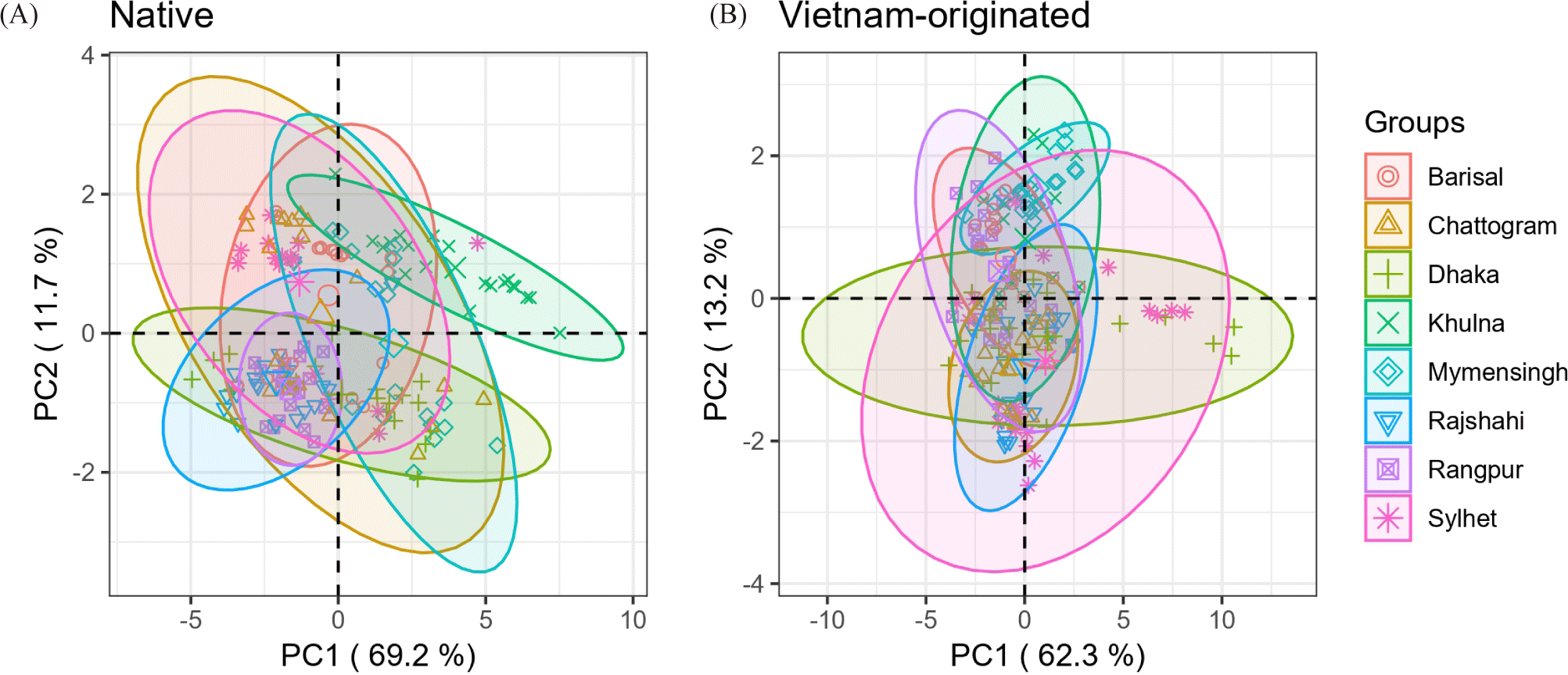
The variation in the unadjusted data of the morphometric parameters related to the source was evaluated individually for the two strains using principal component analysis. The morphometric characters which were used to cluster the populations displayed a high admixture of clusters which were presented with a 95% confidence ellipse (Fig. 4). According to the principal component analysis (Table 5), the intra-species variation of native and Vietnam-originated strains, and three principal components were extracted separately for native and Vietnam-originated populations. The eigenvalues of third principal component (PC3) for both the strains were less than 1 so first principal component (PC1) and second principal component (PC2) were taken into account (Karlis et al., 2003). Around 81% of the total variation among the native groups and 75.5% of the variation of the Vietnam-originated population can be explained by these two principal components. It was reasonable since the Vietnam-originated were cultivated artificially and their feeding habit and other management factors were similar. The PC1 obtained from native explained 69% of the total variation of 10 measurements. However, the PC2’s importance is sometimes constructive in explaining the variation (Delling et al., 2000). Among the native strains, TL had the maximum character loadings in PC1, closely followed by SL and HL. PVFL had the maximum loadings in PC2 and was closely followed by PCFL. Other different length measurements such as BD, DFL, PVFL, AFL, and CFL are factors that may be considered the sources of variation. On the other hand, TL had the highest loading in PC1 and was closely followed by SL and AFL while PVFL had the maximum loading in PC2 and was closely followed by PCFL for Vietnam-originated strains. Hence, the Total AFL was the influential factor that caused the differences among the Vietnam-originated. PCA is considered a valuable tool for finding the morphological characteristics of several species that are regarded as significant sources of variation among populations (Arechavala-Lopez et al., 2012) as well as between populations (Jannat et al., 2022; Nguyen & Duong, 2016; Yakubu & Okunsebor, 2011). A study conducted by Hu et al. (2024) revealed that the morphological characteristics of the Gymnodiptychus dybowskii populations in the Turks River and Manas River were significantly different and the main character associated with PC1 was the terminus of the dorsal fin to the ventral origin of the caudal fin, the main factor related to PC2 was the BD, and the main trait associated with principal component 3 was the terminus of the anal fin to the origin of the anal fin. A principal component analysis study conducted on Channa marulius fish populations of Bangladesh showed that SL, TL, and LDFB of the morphometric measurements were important for the phenotypic variation in populations (Rakhi et al., 2024).
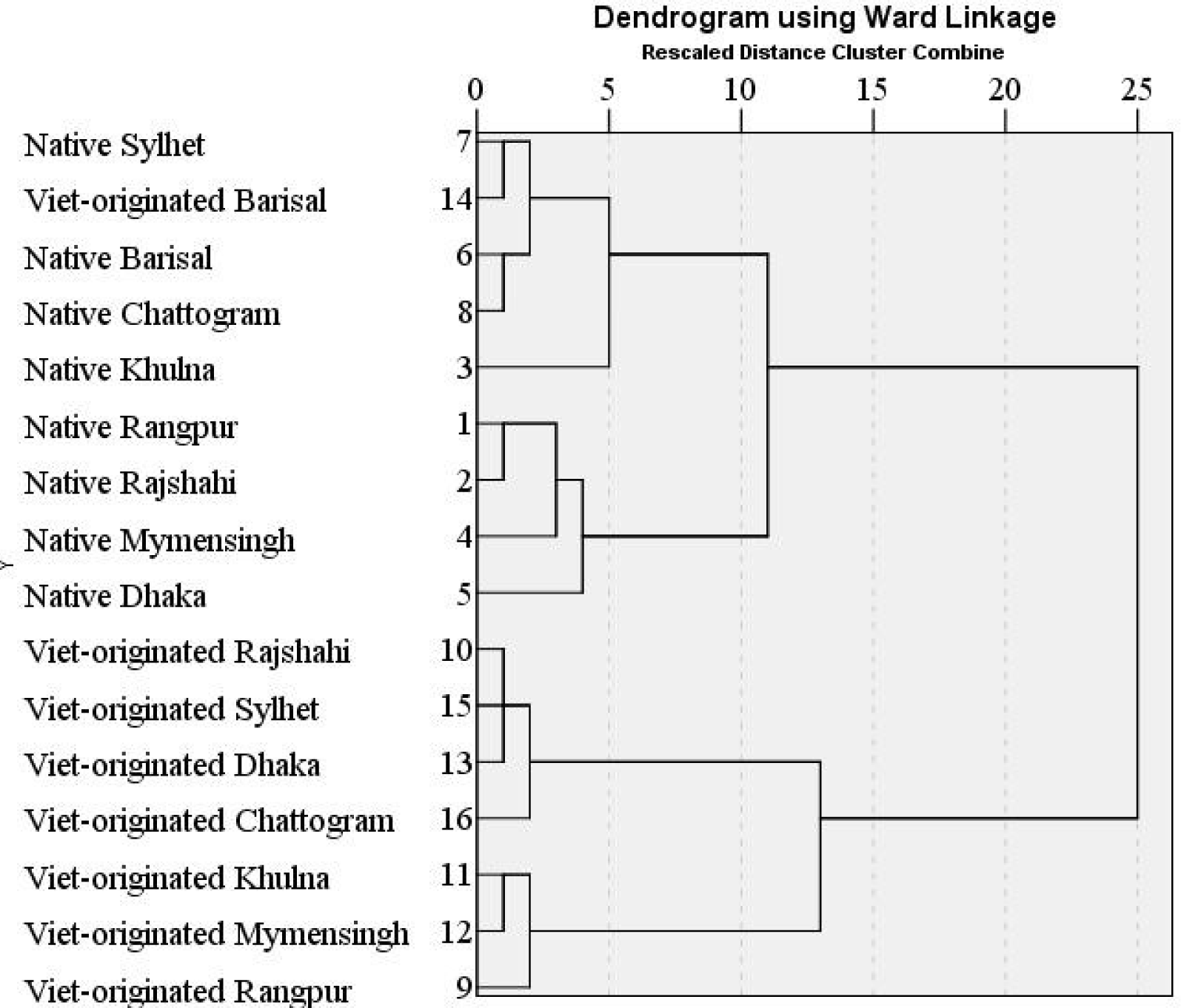
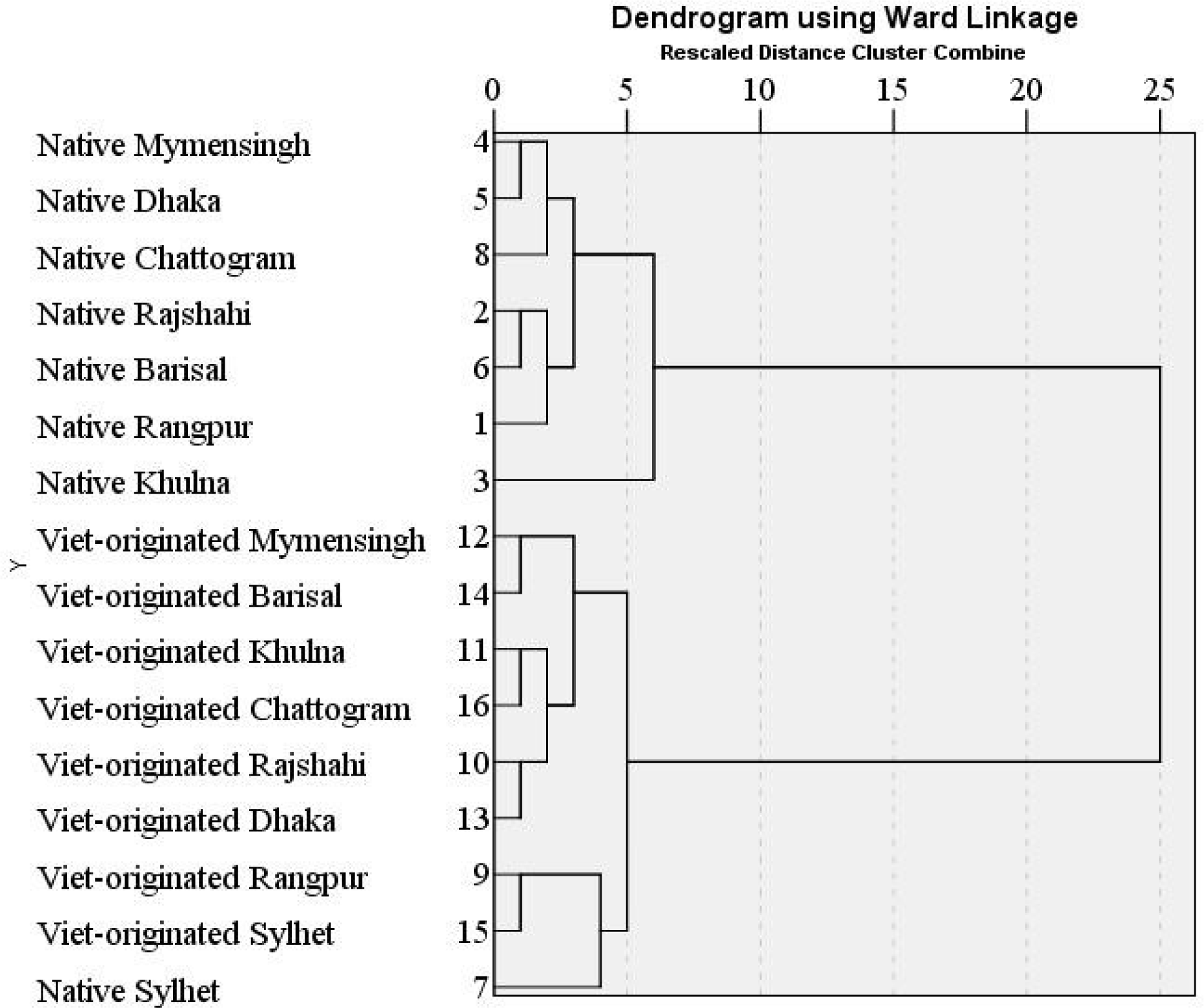
The dendrogram based on the hierarchical cluster analysis using size-adjusted morphometric characters for A. testudineus of the two strains collected from 8 divisions is shown in (Fig. 5). The dendrogram formed two main clusters, all the native A. testudineus and Vietnam-originated A. testudineus collected from Barishal in one cluster and remaining 7 Vietnam-originated A. testudineus population collected from rest of the locations remained in another cluster. It indicates that these two strains were separated concerning morphometric characters though Vietnam-originated A. testudineus from Barisal were miss grouped. The results obtained from hierarchical cluster analysis for meristic characters are presented as a dendrogram in Fig. 6. The two strains clustered differently except the native Sylhet population grouped with the Vietnam-originated population. Therefore, an almost complete separation of the native and Vietnam-originated populations was obtained. Hossen et al. (2017) reported the hierarchical cluster analysis using morphometric characters for indigenous and Thai A. testudineus formed two main clusters separating the two stocks whereas, results obtained from hierarchical cluster analysis for meristic characters did not cluster separately the two populations in the dendrogram. However, Zhao et al. (2023) reported that the clustering relationship diagram of seven golden pompanos (Trachinotus ovatus) populations was successfully divided into three branches.
The diverse morphologies of native and Vietnam-originated climbing perch can be influenced by both abiotic and biotic factors (Novomeská et al., 2013). Fish from the same gene pool, but raised in different aquatic environments have been reported to exhibit distinct differences in morphology (Tibihika et al., 2020; Vehanen & Huusko, 2011). Studies showed that variations in morphometric traits among cultured populations of Oreochromis niloticus have been influenced by genetic differentiation, diet, water depth, stocking density, culture method (pond or cage), habitat, and water quality (Asmamaw & Tessema, 2021; Kwikiriza et al., 2023). However, this study was not intended to investigate the underlying causes of morphometric and meristic variations that occur in varying stocks of the same species and not to determine whether the morphological variations are environmentally induced or due to genetic factors, or both. More research, especially genetic studies are needed to find out the actual causes of variation. This work shows that while the local and Vietnam-originated climbing perch populations can be distinguished using the morpho-meristic approach, a combination of molecular biology techniques is needed for a more precise and scientific assessment of the various populations. The population variations and structure of A. testudineus among these two strains aided by examining the morphological traits and meristic count variations can help in creating breeding and management plans aimed at various ecological populations. Aquaculture practices should be designed in such way that the exotic strains don’t escape in the wild and interfere with the existing gene pool of the native population.
Conclusion
The present study revealed that the native and Vietnam-originated A. testudineus populations found in different regions of Bangladesh differ in all morphological characters and meristic counts except CFL. According to principal component analysis, the two distinguishing morphological features such as TL, and PVFL can be used to differentiate native and Vietnam-originated A. testudineus in Bangladesh. The morphological differentiation of native and exotic populations in Bangladesh can be used for preliminary screening of these two strains at the field level without sophisticated molecular techniques. Besides this basic morphological documentation can be helpful to differentiate different morphotypes of climbing perch in natural water bodies in the future. Thus, correct broodstock of different strains can be identified from nature as per requirement in the future.