Introduction
The false clownfish (Amphiprion ocellaris) is a popular marine ornamental fish, highly valued for its vibrant colors and good adaptability in captive conditions (da Silva et al., 2022). Despite significant progress in breeding this species, challenges remain related to the loss of pigmentation in captive fish, which reduces their commercial value and increases the pressure on natural populations (Calado et al., 2017; Nhan et al., 2019). This loss of pigmentation is thought to be caused by various factors such as stress, water quality, rearing systems, and particularly the content of carotenoid pigments in the diet (Bolker & Hill, 2000; Vissio et al., 2021; Yasir & Qin, 2010). To improve the coloration of cultured fish, techniques such as nutritional supplementation, environmental control, and genetic manipulation have been developed (Lau et al., 2023).
Color plays a crucial role in the survival, reproduction, and commercial value of many ornamental fish species (Gupta et al., 2007; Lau et al., 2023; Sköld et al., 2016). Color is determined by genetic factors, the rearing environment, and pigments, mainly carotenoids, in the diet (Luo et al., 2021). Due to their inability to synthesize carotenoids de novo, fish must obtain them from their diet to develop their characteristic colors (Gupta et al., 2007). Carotenoids also play important roles in fish health, growth, reproduction, and immune function (Elbahnaswy & Elshopakey, 2024; Lim et al., 2023). Among them, astaxanthin stands out for its availability, color-enhancing efficiency, antioxidant properties, and overall health benefits (Lim et al., 2023, 2018). To meet the demand for color enhancement, natural and synthetic pigment supplements have been widely used in aquaculture (Elbahnaswy & Elshopakey, 2024; Lau et al., 2023; Sathyaruban et al., 2021). However, synthetic carotenoids have some drawbacks, such as limited color range, low bioavailability, weak antioxidant capacity, poor color retention, and concerns about consumer acceptance (Gupta et al., 2007; Pereira da Costa & Campos Miranda-Filho, 2020; Trichet & Amaya, 2022). Therefore, the demand for alternative natural carotenoid sources is increasing (Elbahnaswy & Elshopakey, 2024; Sathyaruban et al., 2021). Many studies have demonstrated the positive impact of supplementing natural carotenoids in the diet of aquatic species (Ebeneezar et al., 2020; Nhan et al., 2019; Pham et al., 2014). However, supplementation in the form of raw materials at high rates (15%–30%) can reduce feed quality, digestibility, nutrient absorption, and water quality (García-Chavarría & Lara-Flores, 2013; Nhan et al., 2019; Tran et al., 2022). Therefore, the use of carotenoids in the form of extracts offers many benefits, such as reducing the supplementation rate (0.01%–2.0%), increasing digestibility, absorption, and ease of storage (Ebeneezar et al., 2020; Tran et al., 2022). In particular, animal-derived carotenoids, which primarily exist in the free form of astaxanthin and canthaxanthin, generally have higher bioavailability compared to those from plant sources (Schweiggert & Carle, 2017). Many animal carotenoid sources such as copepods, shrimp, poultry eggs, and mollusks have abundant carotenoid content (0.14–17,200 mg/kg) (Gupta et al., 2007; Schweiggert & Carle, 2017; Tran et al., 2022; Vilgrain et al., 2023). Although widely used in terrestrial animal husbandry, their application in aquaculture, especially for ornamental fish, remains limited. This study aims to evaluate the impact of supplementing natural carotenoids from chicken eggs, shrimp shells, copepod, golden apple snail eggs, and synthetic astaxanthin on the growth, feed utilization efficiency, and coloration of false clownfish. The results will provide valuable information on the potential of animal-derived natural carotenoids in improving the quality of artificially produced ornamental fish.
Materials and Methods
The procedures are conducted in accordance with the national regulations on the use of animals in research in Vietnam, including the Law on Livestock Breeding 2018 and Decree No. 26/2019/ND-CP of the Government, detailing a number of articles and measures implementing the Law on Fisheries, including regulations on the management, protection, and exploitation of endangered, precious, and rare aquatic species. Experiments on marine fish were exempted from ethical approval requirements. However, throughout the study, efforts were made to minimize stress to the fish by providing optimal rearing, care, handling, and sampling conditions.
Animal source materials, including chicken eggs (Gallus gallus domesticus), whiteleg shrimp shells (Penaeus vannamei), copepods (Pseudodiaptomus annandalei), and golden apple snail eggs (Pomacea canaliculata), were collected from different locations in Nha Trang City, Khanh Hoa Province, Vietnam. Ethanol 96% was used as a solvent to extract carotenoids following the method described in Tran et al (2022). Fresh materials (except for chicken eggs and snail eggs, which were boiled and only the yolks were used) (50 g) were mixed with the solvent at a solvent-to-material ratio of 3:1 (mL/g) and homogenized using a blender (Philips HR2118, 600 W, Philips, Amsterdam, the Netherlands). The homogenates were placed in 250 mL glass beakers and subjected to carotenoid extraction using a microwave oven (Sharp microwave, Sakai, Japan) for 180 s. After extraction, the samples were filtered through a filter cloth to separate the extracts from the residues. The residues were further extracted twice (for a total of three extractions) to maximize the recovery of carotenoids from the source materials. The extracts from the three extractions were combined, and the carotenoid content in the samples was determined by colorimetry using a UV-Vis spectrophotometer (Biochrom, Cambridge, UK). The results showed that the total carotenoid contents (TC) in chicken eggs, shrimp shells, copepods, and apple snail eggs were 23.7 µg/g, 52.6 µg/g, 113.3 µg/g, and 81.4 µg/g fresh weight, respectively. The synthetic astaxanthin source used was Carophyll Pink CWS (DSM Nutritional Products), containing 10% astaxanthin. These carotenoid sources were incorporated into the diets of clownfish according to different experimental treatments.
The basal diet (55% crude protein and 12% crude lipid) was formulated to meet the nutritional requirements of marine fish larvae according to their specific needs. The detailed feed composition is presented in Table 1. Four natural carotenoid sources, including chicken eggs (Ch-gg), shrimp shells (Sh-shell), copepods (Cope), and snail eggs (Sn-egg), and a synthetic carotenoid source, astaxanthin (Astax), were supplemented to the basal diet at a level of 250 mg/kg diet. In addition, a control diet (Control) without carotenoid supplementation was tested.
Vitamin premix (mg/kg diet): Vitamin A, 1,000,000 IU; Vitamin D3, 300,000 IU; Vitamin C monophosphate, 10,000 mg; Pantothenic acid, 2,500 mg; Vitamin E, 2,000 mg; Vitamin B3, 2,000 mg; Vitamin K3, 500 mg; Vitamin B1, 500 mg; Vitamin B6, 500 mg; Vitamin B2, 320 mg; Folic acid, 200 mg; Biotin, 20 mg; Vitamin B12, 5 mg; Inositol, 10 mg; Choline chloride, 5 mg.
Mineral premix (mg/kg diet): Zn (ZnO), 4,750 mg; Mn (MnSO4 · H2O), 1,900 mg; Mg (MgO), 1,050 mg; Co (CoCO3), 47.5 mg; Se (Na2SeO2), 47.5 mg; I (Ca(IO2)2 · H2O), 19 mg; P (CaHPO4 · 2H2O), 0.7%; Ca (CaHPO4 · 2H2O), 0.8%; Moisture, 10%; Ash, 2%; Ethoxyquin, 240 mg; Carrier (Dextrose), 86%. Provimi Vietnam, Bien Hoa city, Dong Nai province, Vietnam.
The experimental diets were manufactured following the standard procedures described in detail in Tran et al. (2022), which ensured the quality and consistency of the diets. The main steps of the procedure included weighing the ingredients, grinding, mixing, cooking, cooling, adding carotenoids and vitamins, pelleting, and oven-drying to 10% moisture content. The final feed pellets were 0.8–1.0 mm in size and were stored at –4°C until use.
Juvenile clownfish with an initial size of 3.14 ± 0.02 cm and 0.54 ± 0.02 g were acclimatized in 60-L glass tanks (55 × 35 × 38 cm) at a stocking density of 15 fish per tank for one week. The tanks were part of a recirculating system with a stable water flow rate of 1.5 L/min under natural light conditions. Fish were fed four times a day (7:00, 10:00, 13:00, and 16:00 h). Uneaten feed was collected, dried, and kept separately for feed efficiency evaluation. Tank maintenance included siphoning every two days to remove waste and a weekly 30% water exchange. Optimum water quality was maintained with temperature ranging from 27°C to 31°C, salinity from 32‰ to 34‰, pH from 7.8 to 8.2, dissolved oxygen concentration above 5.0 mg/L, and Total Ammonia Nitrogen (TAN) maintained below 1.5 mg/L. Daily observations were conducted to monitor fish health and environmental conditions.
The study used a completely randomized design to examine the effects of dietary supplementation of carotenoids from different sources on growth, feed utilization, and coloration in juvenile clownfish. Five carotenoid-supplemented diets and a non-supplemented control group were formulated (Table 1), each with three replicates and a duration of 75 days.
At the end of the experiment, all surviving fish were harvested to measure indices of length, weight, survival rate (SR), feed efficiency, and skin color parameters (L*, a*, b*, C*, h*, and Delta E), and carotenoid content in tissues (skin, muscle, and whole body). Fish were starved for 24 h and lightly anesthetized with 0.05% Ethylene Glycol Monophenyl Ether (Merck KGaA, Germany, Merck, Darmstadt, Germany) before sampling to ensure accurate measurements. Fish were then blotted dry for measurements. Six individuals were randomly selected from each tank and stored at –80°C for laboratory carotenoid analysis.
Growth rate assessment parameters:
Survival rate:
Feed utilization efficiency:
where: L1 and L2 are the lengths of fish at the start and end of the experiment (mm); W1 and W2 are the corresponding weights of fish (g); t is the duration of the experiment (days); Standard Deviation of Length (SDL) and Standard Deviation of Weight (SDW) are the standard deviations of fish length (mm) and weight (g); BW is the body weight of fish (g); FI is the amount of feed intake (g); PI is the amount of protein intake (g, 55%); LI is the amount of lipid intake (g, 12%); N1 and N2 are the number of fish at the start and end of the experiment (individuals).
Live fish skin color was measured using a CR-400 colorimeter (KONICA Minolta, Tyoko, Japan) at the midpoint between the dorsal and anal fins, twice on each side of the body. Measurements were taken on all surviving fish at the end of the experiment. The colorimeter is based on the principle of spectrophotometry, using optical filters to simulate the CIE 1931 color matching function, providing information in the CIELAB and CIELCH color spaces. The lightness (L*), green-red coordinate (a*), and blue-yellow coordinate (b*), chroma (C*), and hue angle (h*) were recorded (Hunter & Harold, 1987). Color change (ΔE*) between astaxanthin-supplemented clownfish (L2*, a2*, b2*) and the control group (L1*, a1*, b1*) was calculated using the formula ΔE* = [(L1 – L2)2 + (a1 – a2)2 + (b1 – b2)2]1/2 (Tomasevic et al., 2019).
Total carotenoid content was determined using the UV-Vis spectrophotometry method as described in Tran et al. (2022). Skin and muscle samples (0.25 g/sample), whole body, and feed (1.0 g/sample) were homogenized with acetone and Na2SO4, and then filtered and centrifuged to obtain the extract. The absorbance of the extract was measured to determine the carotenoid content, and the results were expressed as µg/g sample.
The formula for calculating TC is as follows:
where A is the absorbance; V is the volume of the extract (mL); D is the dilution factor; W is the sample weight (g); and E1cm1% is the conversion factor, which is set at 2.100 for edible oil with an absorption wavelength of 485 nm.
Data were analyzed using IBM SPSS Statistics version 22.0 (Armonk, NY, USA). The assumptions of normality and homogeneity of variances were tested prior to applying one-way ANOVA to assess the differences among groups. In the event of a statistically significant difference, Duncan’s Multiple Range Test was conducted to determine the specific differences between groups at a significance level of 5% (p < 0.05). Results are reported as mean values ± SE.
Results
The addition of carotenoids to the diet significantly improved the growth performance of clownfish compared to the control (Table 2). Fish fed diets supplemented with natural carotenoids, especially from copepods and shrimp shells, achieved significantly higher specific growth rates in length and weight of 21.2%–24.2% and 30.4%–34.8%, respectively, compared to the control (p < 0.05). The coefficient of variation in weight (CVW) of fish in the two aforementioned treatments was also lower, indicating greater uniformity than the control (p < 0.05). Notably, the growth performance and CVW of fish in the two treatments supplemented with carotenoids from copepods and shrimp shells were similar and significantly higher than in the treatment supplemented with synthetic astaxanthin (p < 0.05). However, the coefficient of variation in length (CVL) and other indices such as SR and condition factor (CF) did not differ significantly among the experimental treatments (p > 0.05).
Although the daily feeding rate (FR) did not differ significantly, clownfish fed diets supplemented with natural carotenoids from shrimp shells and copepods had significantly lower FCR than the other treatments, ranging from 1.51–1.60 compared to 1.91–1.95 (p < 0.05). Similarly, the other feed utilization efficiency indices, including FER, PER, and LER, were also better in the diets supplemented with carotenoids from shrimp shells and copepods (Table 3).
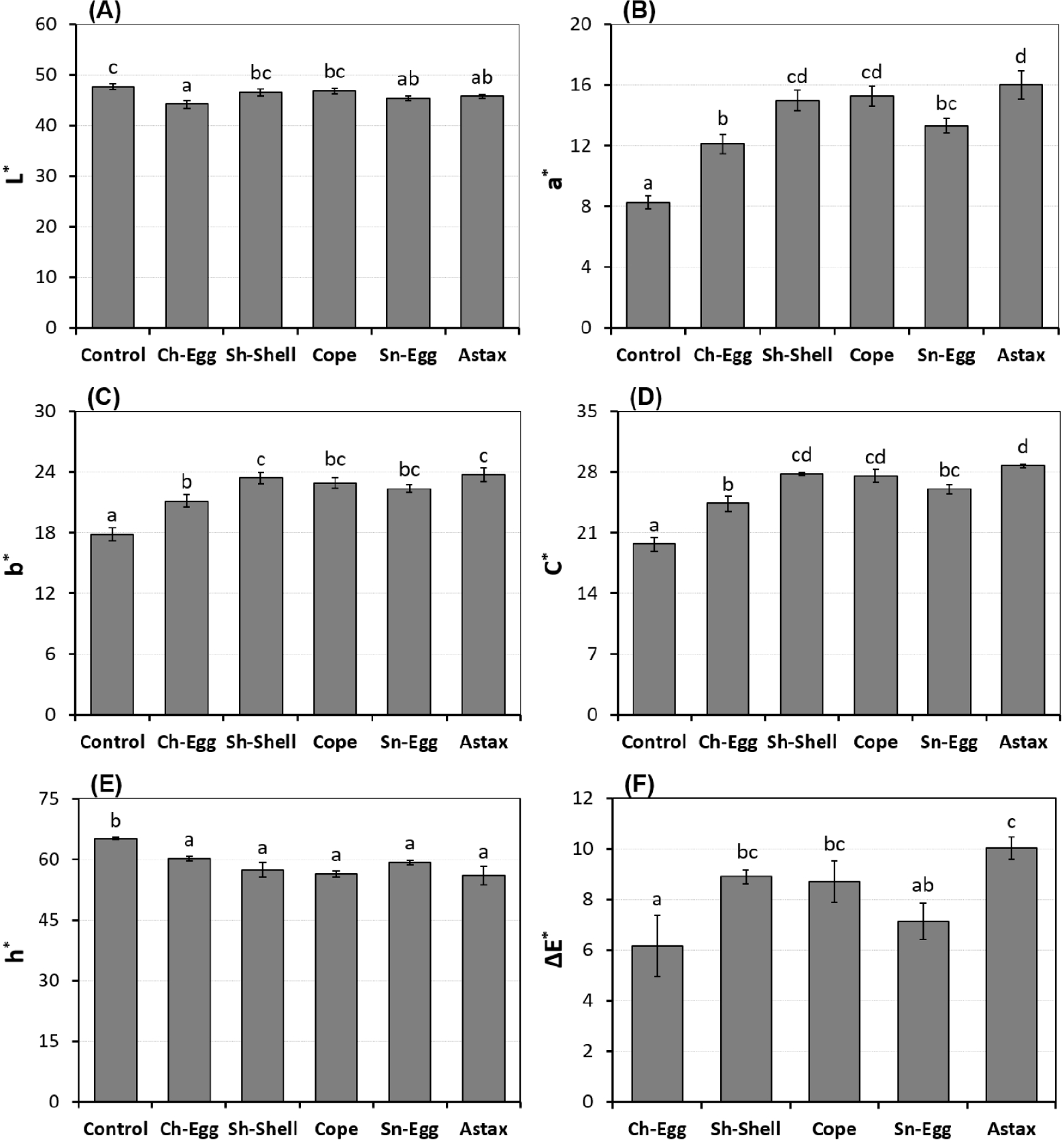
The results of measuring the skin color parameters of clownfish after supplementation with different carotenoid sources are shown in Fig. 1. Specifically, lightness (L*) decreased significantly in the groups supplemented with carotenoids from chicken eggs, snail eggs, and synthetic astaxanthin compared to the control (p < 0.05; Fig. 1A). In contrast, skin redness (a* index) was higher in the carotenoid-supplemented groups than in the control, with the highest values achieved in the treatments supplemented with carotenoid sources from synthetic astaxanthin, copepods, and shrimp shells (Fig. 1B). The increment in skin redness ranged from 80.9% to 93.5% as compared to the control. A similar trend was observed in the measurements of other color indices, including skin yellowness (b*), color saturation (C*), and hue angle (h*) (Fig. 1C–1E). Interestingly, the contrasting trend of high C* and low h* values in the shrimp shell, copepod, and synthetic astaxanthin treatments compared to the other treatments suggests that the skin color of fish in these treatments was more vivid and saturated, and closer to the desired red color in clownfish. The overall color difference (ΔE*) compared to the control was also more pronounced in the group of three aforementioned supplemented treatments than in the other treatments (Fig. 1F).
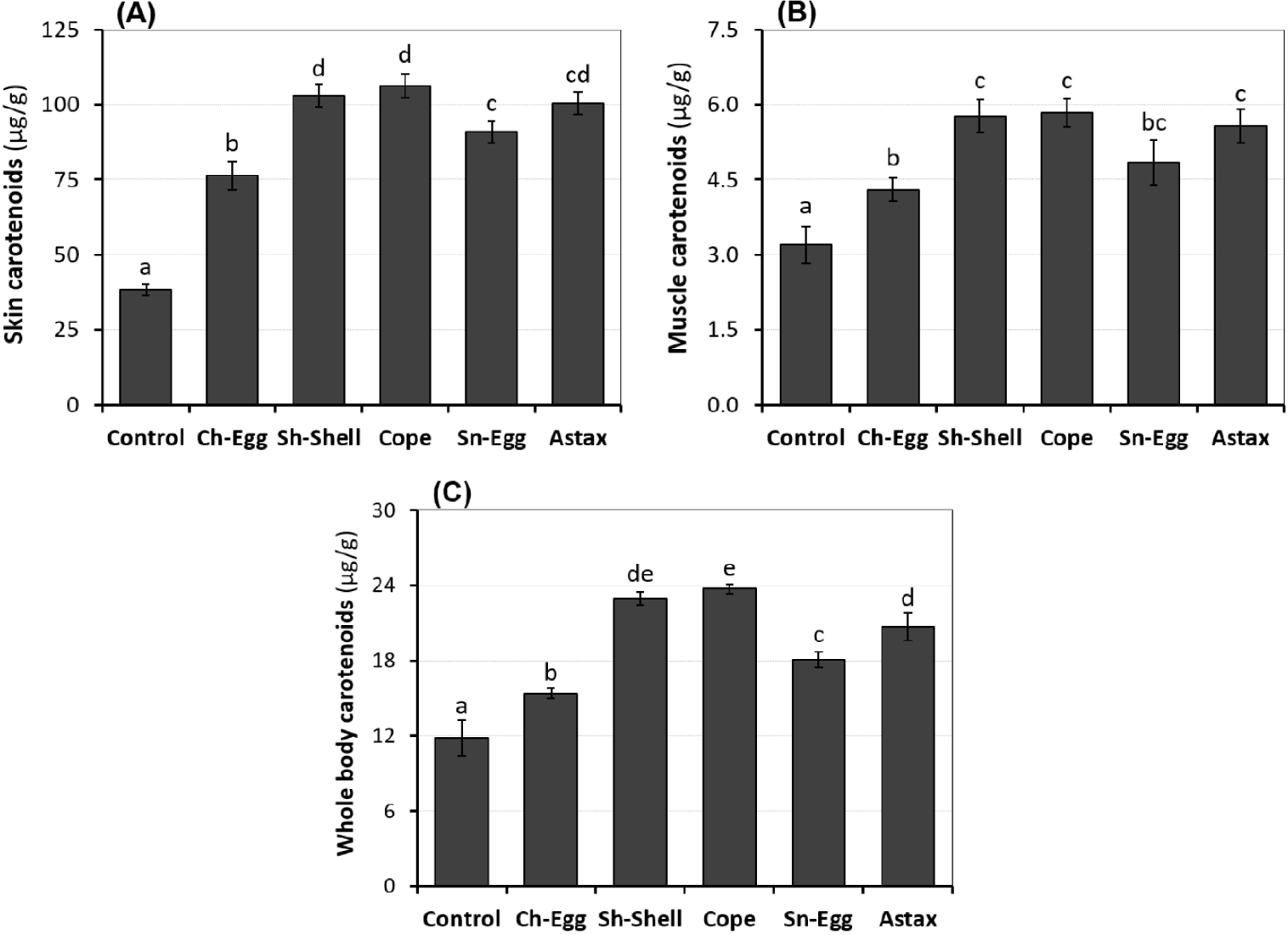
The TC accumulated in the skin, muscle, and whole body of fish is shown in Fig. 2. Specifically, the carotenoid content in the skin increased significantly in the carotenoid-supplemented groups compared to the control group. Among them, the groups supplemented with carotenoids from copepods, shrimp shells, and synthetic astaxanthin achieved the highest results, with an estimated increase of 162%–177% compared to the control (Fig. 2A). Similarly, the carotenoid content in the muscle and whole body of fish also increased significantly in the experimental groups compared to the control (Fig. 2B and 2C). The results demonstrate that supplementing the diet with carotenoid sources, particularly copepods, shrimp shells, and synthetic astaxanthin, effectively enhances the accumulation of carotenoids in fish tissues. This indicates the effectiveness of carotenoid supplementation in the diet to enhance the color quality of ornamental fish in general.
Discussion
Growth and coloration are two of the most important traits of interest in ornamental aquaculture, especially for slow-growing species such as clownfish (Amphiprion spp.). To reach the size of juveniles for commercial farming (3.5–4.5 cm), they require 5–6 months depending on the farming conditions, while this period is only 1.0–1.5 months for the majority of marine fish species farmed for food (Calado et al., 2017; Madhu et al., 2012). Therefore, enhancing growth would shorten the farming time and increase the economic efficiency of the production process. The present study demonstrated that the supplementation of carotenoids from copepods and shrimp shells significantly improved the growth performance and feed utilization efficiency of clownfish, with increases of 21.2%–34.8% and 15.2%–22.6%, respectively, compared to the control (Table 3). This result confirmed the role of carotenoids, especially those extracted from copepods and shrimp shells, in the farming outcomes of fish in general and clownfish in particular.
Recent studies have emphasized the crucial role of carotenoids, which are bioactive substances, in the development, feed conversion efficiency, and overall health of aquatic species (Elbahnaswy & Elshopakey, 2024; Lim et al., 2023). The superior growth in fish supplemented with carotenoids can be explained through several biological mechanisms: for example, they stimulate the activity of digestive enzymes, thereby enhancing nutrient metabolism (Amar et al., 2001; Zhu et al., 2022). In addition, carotenoids also act as antioxidants, reducing oxidative stress and modulating the expression of genes involved in various physiological processes, supporting the efficient use of energy for growth (Fang et al., 2021; Lim et al., 2023). Furthermore, studies by James et al. (2006) and Zhao et al. (2022) suggested that carotenoids have a positive impact on gut structure by improving the characteristics of villi and enhancing the balance of the gut microbiota, which contributes to more efficient digestion and nutrient absorption (James et al., 2006; Zhao et al., 2022).
The findings of the present study are consistent with numerous previous studies investigating the effects of dietary carotenoids in marine fish species such as Pagrus pagrus (Kalinowski et al., 2011), Pseudosciaena crocea (Li et al., 2014), Trachinotus ovatus (Xie et al., 2017), Dicentrarchus labrax (Saleh et al., 2018), and Epinephelus akaara (Song et al., 2021). However, some other studies failed to demonstrate significant growth enhancements from carotenoid supplementation, such as in the cases of P. pagrus (Tejera et al., 2007), Paralichthys olivaceus (Pham et al., 2014), Plectropomus leopardus (Zhu et al., 2022), and Amphiprion percula (Tran et al., 2022). This indicates the complexity and inconsistency in the effects of carotenoids on fish growth. Specifically, the varying results could stem from the variations in factors such as fish species, life stage, diet, source and dosage of carotenoids, as well as different rearing conditions (Elbahnaswy & Elshopakey, 2024; Kalinowski et al., 2011; Lau et al., 2023; Lim et al., 2018). In contrast, the current findings, together with Ebeneezar et al. (2020), showed the benefits of carotenoid supplementation on the growth of A. ocellaris, which was not observed in the works of Díaz‐Jiménez et al. (2021), Nhan et al. (2019), and Seyedi et al. (2013). This inconsistency indicates that there are some factors that have not yet been elucidated that affect the impact of carotenoids, and highlights the need for further research on this topic, such as the influence of environmental factors, nutrient interactions, and genetic factors. Although the present study did not include cytotoxicity assays and histological examinations, the data suggest that carotenoid supplementation did not cause any overt negative effects on the CF and SR of the fish (> 95%). This implies that A. ocellaris can adapt to dietary carotenoid supplementation under the experimental conditions. However, further studies, including cytotoxicity assays and histological examinations, are necessary to comprehensively evaluate the safety and benefits of carotenoid supplementation in this fish species.
The current study demonstrated that supplementing carotenoids from various dietary sources significantly improved the coloration of A. ocellaris, addressing the challenges of discoloration and pigmentation depletion that occur in captive-bred clownfish, which reduce their market value (Nhan et al., 2019). Clownfish tend to lose their brightening color in captivity due to lack of appropriate dietary supply, as well as other factors such as captive stress, water quality, and dietary level or feed consumption (Ebeneezar et al., 2020; Nhan et al., 2019). Previous efforts to enhance and maintain the coloration of cultured ornamental fish through dietary supplementation have been reported (Díaz‐Jiménez et al., 2021; Ebeneezar et al., 2020; Pham et al., 2014; Tran et al., 2022; Yasir & Qin, 2010). In the present study, the supplementation of carotenoids from copepods, shrimp shells, and synthetic astaxanthin significantly enhanced the coloration of clownfish, including both skin color indices and accumulated carotenoid content, compared to the control. In the two most important measured parameters, skin redness (a*) and skin accumulated carotenoid content, the degree of color improvement was estimated to increase by 80.9%–93.5% and 162.4%–177.6%, respectively, in the pigment-supplemented groups compared to the control (Figs. 1 and 2). This confirmed the effectiveness of dietary copepod-, shrimp shell-, and synthetic astaxanthin-derived carotenoids on the coloration of clownfish, which is consistent with previous reports noting similar trends in the effects on this fish species when using different sources of carotenoids, including both natural and synthetic ones (Díaz‐Jiménez et al., 2021; Ebeneezar et al., 2020; Nhan et al., 2019; Seyedi et al., 2013). Compared to the plant-based carotenoid sources that we tested previously (Tran et al., 2024), the color-enhancing efficiency of animal-based carotenoid sources (copepods and shrimp shells) was significantly higher, with increases in a* index and skin accumulated carotenoid content in these two groups compared to the control of 80.9%–93.5% and 162.4%–177.6%, respectively. In contrast, the plant-based carotenoid sources (bell pepper and gac fruit) only achieved increases of 75.7%–89.2% and 89.6%–98.4%, respectively. This result further supports the argument that the color-enhancing efficiency in a particular fish species varies depending on the characteristics of the carotenoid source used and the method of supplementation (Díaz‐Jiménez et al., 2021; Seyedi et al., 2013; Tran et al., 2022; Yasir & Qin, 2010). Future research should focus on identifying the specific factors that influence this variation, such as the chemical structure of the carotenoid, dosage, duration of supplementation, and the absorption capacity of the fish.
In conclusion, supplementation with carotenoids, both natural and synthetic, improved growth, feed utilization efficiency, and coloration in clownfish. Natural carotenoids from copepods and shrimp shells improved growth and feed utilization efficiency better than synthetic astaxanthin, but the color-enhancing effects were similar. Synthetic astaxanthin remains an effective solution for enhancing ornamental fish coloration due to its availability and convenience in use. However, natural carotenoids would be a more sustainable solution for long-term aquaculture, especially considering the issues related to safety, multidimensional effects on the health of the cultured species, and environmental friendliness (Elbahnaswy & Elshopakey, 2024; Trichet & Amaya, 2022). This study supports the use of natural carotenoids to improve the quality and value of cultured ornamental fish, thereby contributing to reducing the pressure on wild reef fish stocks and protecting coral reef ecosystems. However, our study still has several limitations. Firstly, we focused on evaluating the PER and LER based on the protein and lipid content in the feed intake, rather than determining the actual accumulation of protein and lipid in the fish body. Secondly, the optimal dosage and duration of carotenoid supplementation from copepods and shrimp shells need to be further investigated. Thirdly, the mechanisms of carotenoid metabolism, absorption, and accumulation in fish body remain to be elucidated. Lastly, the effects of carotenoid supplementation from copepods and shrimp shells on various health parameters, such as antioxidant capacity, immunity, and stress resistance, warrant further research. Future studies addressing these aspects would provide a more comprehensive understanding of the benefits and limitations of natural carotenoid supplementation in ornamental fish nutrition.
