Introduction
Silver pompano (Trachinotus blochii) is one of the most important commodities in the fisheries sector in Lampung. Silver pompano has good market prospects in the Asia-Pacific region and has high economic value (Linga Prabu et al., 2022). The high price of silver pompano encourages continued fishing efforts that can be detrimental to the sustainability of the population. This damage is caused by inappropriate methods of fishing in nature (Cao et al., 2020). There are concerns that high levels of fishing from the wild will lead to extinction (Di Muri et al., 2018). Opportunities in the domestic and export markets are massive, thus the urgent necessity to start an aquaculture business that includes silver pompano.
The Demonstration of Farming (Dem-farm) of silver pompano has been successfully developed by the Lampung Marine Aquaculture Center with satisfactory results (Haryanto et al., 2021). The effort to preserve and develop silver pompano is domestication, namely through hatchery activities (Shen et al., 2021). The success of marine aquaculture depends on high production levels, which are influenced by high growth rates and high survival rates (SRs). Growth and survival rates are affected by feed supply, environmental quality, pests, and diseases (Calleja et al., 2022).
The larval stage is very vulnerable in the life cycle of fish. It is characterized by high mortality and susceptibility to pests and diseases (Malzahn et al., 2022). High mortality in larval fish is often caused by feeds that are not suitable for open-mouth feeding and difficulties in obtaining a continuous feed supply (Zhao et al., 2022). A good feed for larvae depleted of yolk is high in nutrients and protein for growth. Malzahn et al. (2022) suggest that the feed should be a natural feed, which in addition to being a source of carbohydrates and protein, also has essential fatty acids and minerals that are complete for fish larvae, in addition to being easy to digest and not polluting the aquatic environment and larval rearing medium.
The natural feed used is Brachionus. Brachionus belongs to the phylum Rotifera, class Monogononta, order Ploima, family Brachionidae, genus Brachionus, and species Brachionus plicatilis (Müller & Fabricii, 1786). Rotifers are white, cup-shaped, and have flagellates in their mouths that move actively. This species is of great commercial importance and valuable. Its small size, shape, and slow swimming speed make it suitable as live food for the larvae of several marine species (Granada et al., 2022; Lawrence et al., 2012). According to Granada et al. (2022), another potential advantage of rotifers is that they proliferate very easily and are highly nutritious, rich in fatty acids and antibiotics.
Rotifer is a non-selective filter feeder, which does not selectively filter all available feed, so eicosapentaenoic acid (EPA) and decosahexaenoic acid (DHA) extracted from black soldier fly (BSF) added to the rearing medium will be eaten by rotifer. Rotifer which has increased its nutritional content is expected to meet the nutritional needs of silver pompano larvae for growth performance. Based on this, it is necessary to research the extraction of EPA and DHA from maggot BSF as a natural feed enrichment to improve the survival performance of silver pompano larvae.
Materials and Methods
Ethical approval was not required for this study; however, samples were collected as per the standard sample collection procedure.
Sampling maggot BSF was conducted in February 2023, BSF samples were collected at the maggot BSF Breeding Group, Sadar Sriwijaya Village, Bandar Sribhawono, East Lampung (5°16’28.7 ”S 105°42’43.7”E). BSF samples were handled at the Laboratory Fisheries and Marine University of Lampung (5°21’59.0”S 105°14’30.5”E). Extraction of EPA and DHA of BSF was conducted at the Agricultural Product Quality Testing Laboratory, Faculty of Agriculture, University of Lampung (5°21’57.4 ”S 105°14’34.6”E) and analysis of growth performance of silver pompano larvae was conducted at the Marine Aquaculture Center, Lampung (5°31’39.5”S 105°14’56.1”E) (Fig. 1).
This study used 15-day-old BSF, samples were taken using a hand glove, stored in a container, and brought to the Laboratory of Fisheries and Marine at the University of Lampung. Maggot BSF were then frozen in the refrigerator at –18°C for 24 hours. The process of producing maggot powder was carried out by thawing the frozen maggot, washing the maggot BSF until it was cleaned, and then drying with an oven at 45°C for one hour and grinding the maggot BSF with a blender, the sample that had been mashed, put in plastic, and labeled, the sample was stored at room temperature (Fig. 2). The extraction of EPA and DHA from maggots BSF was carried out at the Agricultural Product Quality Testing Laboratory, Faculty of Agriculture, University of Lampung.

The extraction of fat from maggot BSF was carried out by the Folch method using the mixture of chloroform-methanol (2:1). A total of 400 g of mashed maggot BSF was dissolved using chloroform and methanol for the extraction of EPA and DHA fatty acids. The first step was to trans-esterify the fatty acid extraction products by the addition of 15% boron tri fluoride solution in methanol which was heated at 60°C for 30 min. Then the solution was cooled and added with n-hexane, shaken to dissolve all in n-hexane. The mixture was allowed to stand for ± 10 min until two layers were formed. The upper layer of methyl ester in n-hexane was separated from the lower layer of methanol and then injected into the gas chromatography. The retention times of the expected components (EPA and DHA) of the chromatograms obtained were determined by comparing them with the retention times of EPA and DHA standards for which chromatograms had previously been made. To confirm the presence of EPA and DHA, before the sample was injected into the gas chromatograph, standard EPA and DHA methyl esters were added alternately (spiking method).
The containers and equipment to be used were sterilized with 10 ppm chlorine and then filled with 40 L of sterile seawater. Then sterilized again with 10 ppm chlorine and kept for 24 hours. The total containers used in B. plicatilis culture were 3 tanks with a volume of 1,500 L and filled with 1,000 L of seawater. The container was first inoculated with 100 L of Chlorella sp. B. plicatilis seedlings were spread into the container with an initial density of 50 ind/mL. After the density reached 100–350 ind/mL (3–5 days after inoculation), B. plicatilis was ready to be harvested for enrichment or fed directly to the larvae following the treatment. The density of B. plicatilis fed to fish was 13 ind/mL per tank.
The enrichment of B. plicatilis in this study refers to Jusadi et al. (2015). The first step was to spread B. plicatilis from mass culture into a 20 L container with a density of 500 ind/mL. In the second step, several doses of enrichment were mixed into the container that contains B. plicatilis. The enrichment process was conducted for 6 hours at 28°C and aerated. In the third step, B. plicatilis with a size of 100–175 µm that has been enriched with EPA and DHA is filtered using a 50 µm plankton net (mesh size 300), then washed with seawater to be given to silver pompano larvae at 5:00 am, 2:00 pm, and 9:00 pm.
The experimental design of combining phytoplankton and B. plicatilis enrichment on the growth and survival of silver pompano larvae (The initial length of 5-day-old silver pompano larvae is 0.25 cm) is 5 treatments with 3 replications. We feed rotifers for 30 days post-separation of egg yolk. Afterward, we continued to feed the larvae with brine shrimp nauplii (Artemia salina) a twice day for 7 days. After that, we feed pellets based on the required needs of the silver pompano larvae. The tank size used in this study is 20 × 20 × 20 cm, with water conditions that are monitored periodically. The experimental design is as follows:
-
B. plicatilis (No enrichment; 0 ppm)
-
B. plicatilis with EPA & DHA enrichment (100 ppm)
-
B. plicatilis with EPA & DHA enrichment (300 ppm)
-
B. plicatilis with EPA & DHA enrichment (500 ppm)
-
B. plicatilis with EPA & DHA enrichment (700 ppm)
The duration of the experiment in this study was 30 days post-separation of egg yolk. The frequency of data collection, such as measuring fish length, was conducted weekly by randomly sampling one larvae in each tank and measured using a ruler. At the end of the experiment, the number of live larvae was calculated to determine the SR.
The total of silver pompano larvae samples used in this study was 20 larvae per tank. A total of 15 tanks were used. Thus, the total larval used in this study was 300 larvae. Samples of silver pompano larvae were reared at the Marine Aquaculture Center in Lampung Province, Indonesia, for growth performance examination.
The main parameters in this study consisted of two aspects, namely growth in the form of absolute length measurements, and SR. The growth rate is the absolute length of growth. The equation to determine the absolute length growth rate during rearing uses the equation from Mulqan et al. (2017) as follow:
PM = Absolute length (cm)
Lt = Final average length (cm)
Lo = Initial average length (cm).
The SR is calculated using the equation Muchlisin et al. (2016) provided.
SR = Survival (%)
Nt = Number of fish alive at the end of observation (fish)
No = Number of fish at the beginning of observation (fish).
Supplementary parameters in this study measure water quality. Water quality parameters observed in this study include salinity using a refractometer, temperature measured using a thermometer, and pH measured using a pH pen.
Analysis data of the growth performance includes absolute length growth data and SR of silver pompano. Absolute length growth is a measure of length at a time. The method of measuring the total length of the larvae is conducted by the distance between the tip of the mouth to the tip of the tail fin using a ruler which is expressed in centimeters or millimeters. Data are reported in statistical analysis using one-way analysis of variance (ANOVA). The significance test was carried out using the last significant difference (LSD) test to determine the significance level which was analyzed using Microsoft Excel. Statistical analysis was conducted to determine the effect of the addition of EPA & DHA extract from Maggot BSF on the enrichment of Brachionus plicatilis for the growth performance of Silver Pompano.
Results
Based on the results of the study, data showed absolute length growth (cm), SR (%), and water quality parameters during rearing between treatments A (no enrichment), B (EPA and DHA enrichment of B. plicatilis 100 ppm), C (enrichment of EPA and DHA B. plicatilis 300 ppm), D (enrichment of EPA and DHA B. plicatilis 500 ppm), and E (enrichment of EPA and DHA B. plicatilis 700 ppm). Total of EPA and DHA content of 5.05% EPA and 1.2% DHA.
The results of body length measurements of silver pompano larvae that treatment C with a dose of EPA and DHA from maggot BSF extract of 300 ppm (0.3 mL/L) has the highest absolute length growth of 2.11 ± 0.04 cm, then treatment B with a dose of 100 ppm (0.1 mL/L) EPA and DHA from maggot BSF extract showed the second highest absolute length growth with a result of 2.03 ± 0.05 cm, The treatment that has the third largest absolute length growth value is the treatment with a dose of 500 ppm (0.5 mL/L) which is treatment D with absolute length growth of 1.97 ± 0.09 cm, then for treatment A with EPA and DHA from maggot BSF extract showed absolute length growth of 1.81 ± 0.02 cm, and the lowest absolute length growth was 1.70 ± 0.16 cm obtained in treatment E with a dose of 700 ppm (0.7 mL/L). Based on these results, it is known that the best dose that can be used in the enrichment of B. plicatilis using EPA and DHA from maggot BSF extract is a dose of 300 ppm (0.3 mL/L).
Furthermore, to determine the effect of different doses of each treatment on the absolute length growth of silver pompano larvae, the data from the study were tested with analysis of variance (ANOVA). The results of ANOVA are shown in Table 1.
| Source of variance | SS | df | MS | Fcal | F0.05 |
|---|---|---|---|---|---|
| Treatments | 0.33082333 | 4 | 0.08270583 | 11.50023171) | 3.47804969 |
| Errors | 0.07191667 | 10 | 0.00719167 | NA | NA |
| Total | 0.40274 | 14 | NA | NA | NA |
The results of the ANOVA test showed that the Fcal was 11,500, meaning that Fcal is greater than alpha 5% or 0.05 with an Ftab of 3.478 (Fcal > Ftab 5%). It indicated a significant difference among the treatments on the provision of different doses of EPA and DHA on the absolute length growth of silver pompano larvae. Based on these results, it is necessary to analyze by LSD test to determine the differences in each treatment. LSD test results are shown in Table 2.
| LSD test | ||||||
|---|---|---|---|---|---|---|
| 0.05 | 0.15428 | |||||
| Treatment | Means | Notation | ||||
| C | 2. 1067 | a | ||||
| B | 2.0283 | 0.0783 | ab | |||
| D | 1.9733 | 0.1333 | 0.0550 | a | ||
| A | 1.8050 | 0.3017 | 0.2233 | 0.1683 | bcd | |
| E | 17017 | 0.4050 | 0.3267 | 0.2717 | 0.1033 | cd |
The results of the LSD test showed that treatment C with a dose of 300 ppm (0.3 mL/L) was the best treatment in enhancing the absolute length growth of silver pompano larvae with an average of 2.11 cm and significantly different from treatments A and E. In addition, treatment E with a dose of 700 ppm (0.7 mL/L) was the treatment that showed the lowest absolute length of 1.70 cm and was significantly different with treatments C, B, and D, but not significantly different with treatment A.
The research assumed that there is a significant effect on the enrichment of B. plicatilis using EPA and DHA extracts from maggot BSF on the absolute length growth of silver pompano larvae. Based on the results of this study, there are significant differences for each treatment, so it can be stated that the results of this study are valid.
SR is the ratio of the total fish alive at the end of the rearing period with the total at the beginning of rearing represented in percentage (%). The results of SR for each treatment are shown in Fig. 3.
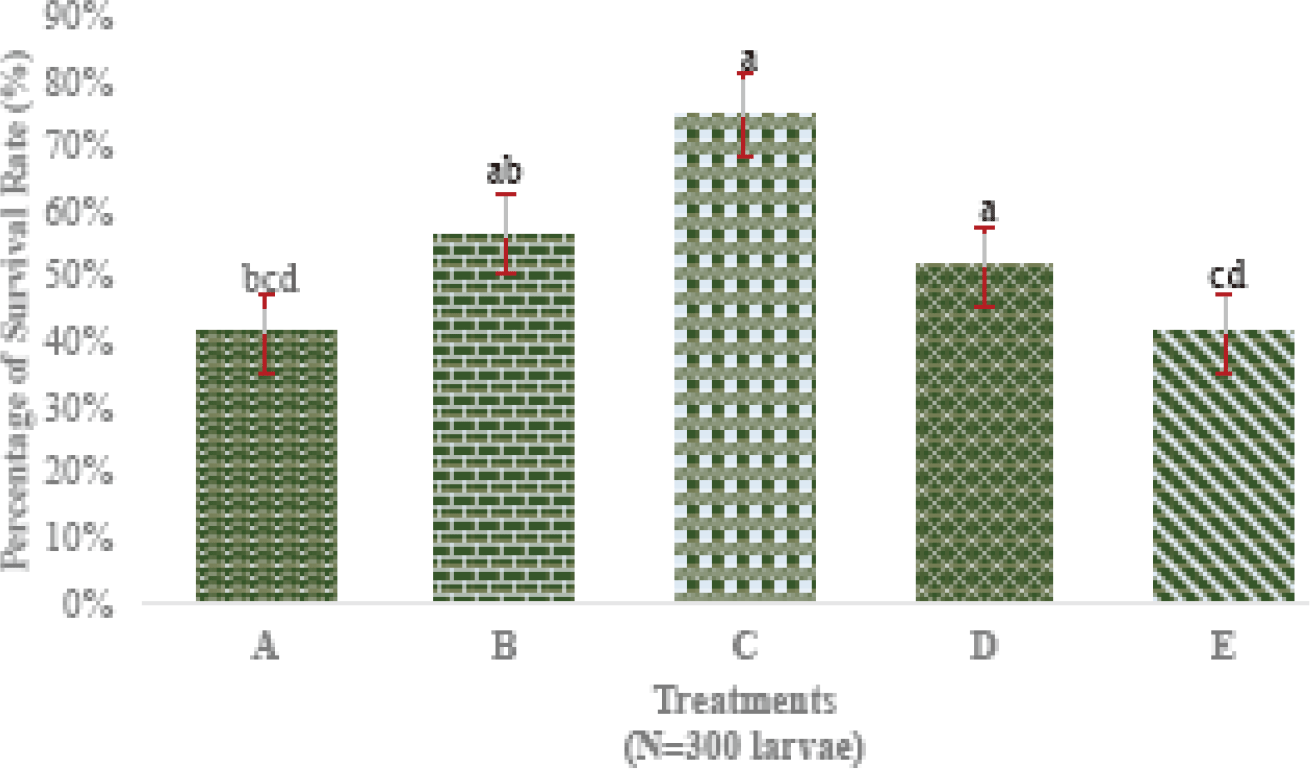
The results showed that treatments A and E with doses of EPA and DHA from the extract of maggot BSF were 0 ppm (0 mL/L) and 700 ppm (0.7 mL/L) respectively had the lowest SR with a mean of 42% ± 1.53. In comparison, other treatments such as treatment B with a dose of 100 ppm (0.1 mL/L) obtained a 57% ± 0.58, and treatment D with a dose of 500 ppm (0.5 mL/L) had a mean SR of 52% ± 1.53. Treatment C with a dose of 300 ppm (0.3 mL/L) has the highest SR which is 75% ± 1.00. Based on these observations, it is known that a dose of 700 ppm (0.7 mL/L) in treatment E has an impact on reducing the SR of silver pompano larvae. It is due to the high oil content that affects the health of silver pompano larvae. Furthermore, the data were analyzed with ANOVA to determine the effect of different doses of each treatment on the SR of silver pompano. The results of the ANOVA test are shown in Table 3.
| Source of variance | SS | df | MS | Fcal | F0,05 |
|---|---|---|---|---|---|
| Treatments | 90.6666667 | 4 | 22.6666667 | 17.89473681) | 3.47804969 |
| Error | 12.6666667 | 10 | 1.26666667 | NA | NA |
| Total | 103.333333 | 14 | NA | NA | NA |
The results of ANOVA tests showed that F calculated of 17.894 which means that F calculated is greater than alpha 5% or 0.05 with the F table of 3.478 (Fcal > Ftab), it indicates that there is a very significant difference between the treatments on the dosing of EPA and DHA from maggot BSF extract on the SR of silver pompano larvae. Based on these results, it is necessary to analyze by LSD test to determine the differences in each treatment. LSD test results are shown in Table 4.
| LSD test | ||||||
|---|---|---|---|---|---|---|
| 0.05 | 2.04752 | |||||
| Treatment | Means | Notation | ||||
| C | 0.75 | a | ||||
| B | 0.57 | 0.18 | ab | |||
| D | 0.52 | 0.23 | 0.050 | a | ||
| A | 0.42 | 0.328 | 0.148 | 0.098 | bcd | |
| E | 0.421 | 0.329 | 0.149 | 0.099 | 0.0010 | cd |
The LSD test results showed that treatment C with a dose of 300 ppm (0.3 mL/L) was the best treatment in increasing the SR of silver pompano larvae with an average of 75% and significantly different from treatments A and E because both treatments were the treatments that showed the lowest survival of 42% and significantly different from treatments C, B, and D. This study assumes that there is a significant effect on the enrichment of B. plicatilis using EPA and DHA extracts from BSF maggot on the SR of silver pompano larvae.
This study assumes that there is a significant effect on the enrichment of B. plicatilis using EPA and DHA extracts from BSF maggot on the SR of silver pompano larvae. Based on the results of this study, there are significant differences for each treatment, so it can be stated that the results of this study are valid.
Water quality measurements in this study consist of temperature, pH, and salinity. Data on the results of water quality measurements are shown in Table 5.
| Water quality | Means | SNI 7901.4:2013 | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| pH | 7.7 | 7.7 | 7.7 | 7.7 | 7.7 | 7.5–8.5 |
| Salinity (ppt) | 30 | 30 | 30 | 30 | 30 | ≥ 28 |
| Temperature (°C) | 29 | 29.1 | 29 | 29.6 | 29.3 | 28–32 |
On the table, it is known that the pH has an average of 7.71 to 7.76. Based on these results, it is recognized that the pH is at an optimal level for the rearing of silver pompano larvae. These results are under The Indonesian National Standard (SNI) 7901.4: 2013 concerning the production of silver pompano (T. blochii, Lacepede) which states that the optimal pH threshold in the rearing of silver pompano larvae is between 7.5 to 8.5, so it is stated that the pH in this study is at the optimal threshold.
As for salinity, it has a similar average of 30.0 ppt. Based on these results, it is known that the salinity of the rearing media is in the optimal range. These results are under SNI 7901.4: 2013 on the production of silver pompano (T. blochii, Lacepede) which states the optimal threshold of salinity in the rearing of silver pompano larvae is at least 28 ppt, so it is declared that the salinity in this study is at the optimal threshold.
The water temperature measured in the morning and evening has an average of 29°C to 29.6°C. Based on these results, it is known that the water temperature of the silver pompano larvae rearing media is in the optimal range. These results are under SNI 7901.4: 2013 on the production of silver pompano (T. blochii, Lacepede) which states that the optimal temperature threshold in the rearing of silver pompano larvae is from 28°C–32°C, so it is confirmed that the temperature in this study is at the optimal threshold.
Discussion
Based on the results of measuring the total length of silver pompano larvae, shows that growth increases with maturation. This follows the advice of Sekar et al. (2021), that growth is defined as the process of changing size (weight, length, or volume) over some time (individual level), and subsequently, Shao & Zeng (2020) stated that growth rate is both absolute growth and relative growth. Absolute growth is the growth in length or weight in a certain period, while relative growth is the growth in length or weight achieved at a certain time concerning the length or weight of a specific period.
Growth performance in larvae is also affected by the management of feeding. Providing feed to the larvae in this study was done by a combination of natural and commercial feeds. The natural feed was B. plicatilis enriched with EPA and DHA from maggot BSF. The commercial feed was in the form of pellets that were adjusted to the mouth gap of the larvae. The feeding time is three times a day at 05.00 am, 02.00 pm, and 09.00 pm. According to Amenyogbe et al. (2022), the type of feed provided influences the activity of digestive enzymes, whereas certain types of feed can increase the activity of digestive enzymes in larvae. The provision of commercial feed must be adjusted to the physiological readiness of the larvae because commercial feed consists of nutrients that have complex molecular structures and are devoid of enzymes, thus requiring the availability of enzymes to digest them. The ability of fish to digest feed is highly dependent on the completeness of the digestive organs and the availability of digestive enzymes. The development of the digestive tract is gradual and after the fish grows to a suitable size or age, its digestive tract will complete.
Based on the results, it is found that treatment C with a dose of 300 ppm (0.3 mL/L) is the best treatment. It is related to the report of Mohan et al. (2022) which states that the oil from maggot extract has the potential as a source of animal fat with a high value because it contains fatty acids (HUFA) consisting of AA (Arachidonic Acid) of 0.075%, EPA of 0.03% and DHA with a total percentage of 0.012%. In addition, Mohan et al. (2022) also explained that the oil from maggot extract contains protein with a percentage of 68.7% and fat of 15.98%. According to previous studies conducted by Widianingrum et al. (2021), the essential fatty acids contained in magot flour are linoleic acid (EPA and DHA) 13.39%. The content of linoleic acid (EPA and DHA) in feed can function as an antimicrobial and immunomodulator (Widianingrum et al., 2019). Linoleic acid (EPA and DHA) is known to affect microbes in the small intestine. Fat in maggots using organic waste feed media contains 60% fatty acids. According to Skřivanová et al. (2005), monolaurin compounds derived from linoleic acid have antibacterial properties on Staphylococcus aureus and Escherichia coli. The results of the analysis of linoleic acid content (EPA and DHA) of maggot flour in Harlystiarini’s research (2017), which used maggots of the same age in this study, namely 15 days, and used the rendering process to break down maggot fat, contained 49.18% linoleic acid (EPA and DHA). According to Pham et al. (2023), larvae fed with DHA content will have an impact on increasing immunity, and the presence of DHA and EPA content will function as the main component of membrane phospholipids that assist cell membrane fluidity to increase cell membrane fluidity in the larval body which can increase the survivability of larvae.
The low SR in treatment E with the highest dose of 0.7 mL/L is due to the dose of oil that is too high and has the natural properties of oil that cannot dissolve in water, causing star pomfret larvae to be disturbed by the high dose of EPA and DHA oil extracted from maggot BSF. This is related to the report of Jadhav & Annapure (2021) that fats and oils are one of the groups that belong to the lipid group, which are organic compounds that have one distinctive property, namely insoluble in water. Related to the SR in marine fish larvae by Sneddon et al. (2016) state that the factors that cause abnormalities in marine fish larvae produced from hatcheries such as density, egg handling, and larval rearing methods, the environment, including temperature, oxygen, light intensity, pollutants in water, salinity, genetics, disease, and nutritional factors.
Drizo & Shaikh (2023) stated that water quality management for aquaculture is very important because water is a living environment for aquaculture organisms. In conditions of poor water quality, a lot of energy is used for the process of physiological adaptation of the fish body to the environment. This results in the proportion of energy stored in the body will be less. In addition, disturbed physiological conditions cause a decrease in feed consumption by fish to minimize the energy used, so that the fulfillment of the energy needed comes from nutrient reserves stored in the fish body.
Conclusion
The application of EPA & DHA oil from maggot BSF with different doses of B. plicatilis enrichment significantly affects the growth of silver pompano larvae. The results showed that the optimal dose of EPA & DHA oil was 300 ppm (0.3 mL/L) in treatment C with an average absolute length growth of 2.11 cm and a SR of 75%, meanwhile for the physical and chemical parameters of water quality, namely pH, temperature, and salinity during the rearing period of pomfret larvae were categorized as optimal.
