Introduction
The golden trevally (Gnathanodon speciosus) is known as a commercially valuable fish, and has become a new species for aquaculture in Vietnam due to successful artificial reproduction in captivity in the hatcheries, its fast growth, and high market value (Ngo et al., 2023). It is found to distribute widely in the tropical and subtropical waters in the Indo-Pacific and Atlantic Oceans (Kongkeo et al., 2010; Ngo et al., 2023). This species is considered valuable both as a food source and an ornamental fish. According to many previous studies, carotenoids play a vital role in the diet of fish, enhancing their growth and development as well as contributing to color enhancement (Gupta et al., 2007). The consumption of carotenoid-rich feed has been linked with numerous health benefits, such as anti-oxidation, enhanced immunity, stress resistance, reproduction, and resistance to bacterial and fungal diseases (García-Chavarría & Lara-Flores, 2013; García-Romero et al., 2014). In nature, fish are unable to biosynthesize carotenoids; however, they can accumulate these pigments in their bodies through the consumption of phytoplankton and some microalgae (Boonyapakdee et al., 2015; Gupta et al., 2007). Conversely, when fish are cultured in high-density, captive conditions without the supplementation of dietary carotenoids, this can lead to faded pigmentation and slow growth, which in turn can decrease the commercial value of the fish (Maiti et al., 2017; Wassef et al., 2010).
Color in the skin and flesh of fish (yellow, red, orange, pink and other colors), is a highly attractive physical characteristic of aquatic organisms; and can be maintained through diets supplemented with carotenoids. A lack of coloration is one of the most significant issues for aquarium species and fish producers. Consequently, there has been a move to develop various sources of carotenoids from both synthetic and natural products to enhance coloration in aquatic organisms (Luo et al., 2021). The challenge, however, lies in the high cost of synthetic components for farmers and growing concerns regarding the safety of artificial colorants (García-Chavarría & Lara-Flores, 2013). This has prompted researchers to explore more natural and cost-effective sources of carotenoids, such as agricultural by-products (including fruits, flowers, roots, vegetables…), which have been recognized as potential sources of mixed carotenoids (García-Chavarría & Lara-Flores, 2013; Gupta et al., 2007; Maiti et al., 2017; Singh et al., 2021).
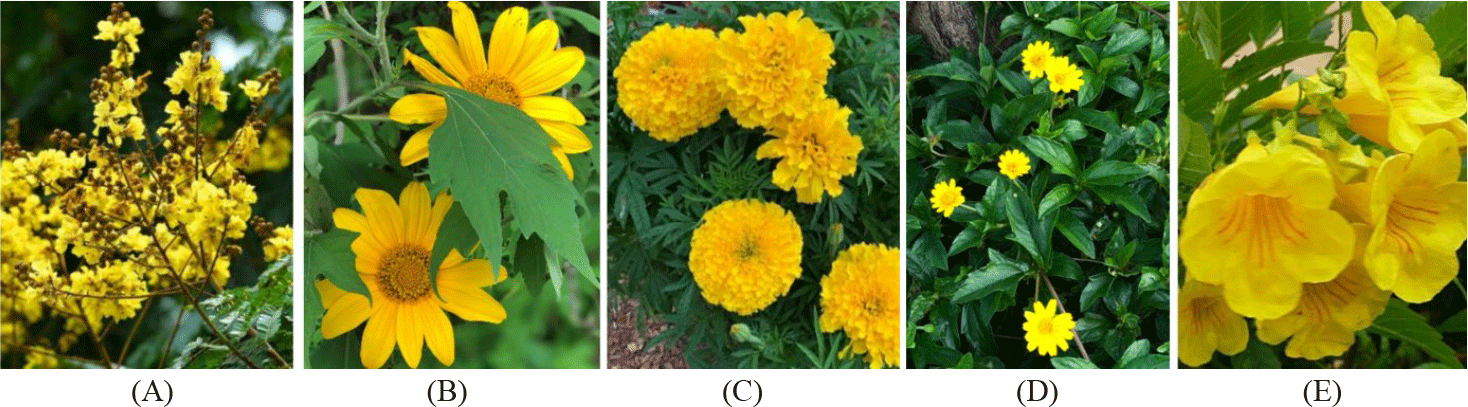
Carotenoid sources from flowers are particularly interesting due to their widespread availability and cost-effectiveness. Carotenoids extracted from flower petals include carotenes, which impart red hues, and xanthophylls such as lutein, zeaxanthin, β-cryptoxanthin, and canthaxanthin, which provide yellow colors (Ratananikom et al., 2021; Yeşilayer et al., 2020). It has been observed that the carotenoid composition in flower petals differs markedly from that in leaves, leading to a rich diversity in petal carotenoid content (Ohmiya, 2011). Consequently, flower petals could be considered a promising source of carotenoids (Ratananikom et al., 2021). Notably, the petal extracts of the leopard tree (Caesalpinia ferrea), wild sunflower (Tithonia diversifolia), marigold (Tagetes erecta), yellow bells (Tecoma stans), and Wedelia chinensis (Wedelia chinensis), which are known for their xanthophylls content, yielding yellow-orange pigments, have been identified to contain compounds like lutein, violaxanthin, zeaxanthin, β-cryptoxanthin, and β-carotene (Maniat et al., 2014; Wassef et al., 2010).
To the best of our knowledge, while there are numerous studies on the impact of marigold flowers on different fish species, information on the effects of the carotenoids from the aforementioned five flower species on the growth of the golden trevally is scarce. Therefore, our study aimed to evaluate the influence of carotenoids extracted from these five flower species on skin pigmentation and growth performance in the golden trevally. The outcomes of this research may underscore the importance of investigating a variety of plants and agricultural by-products as viable, economical, and safe alternatives for aquaculture farmers seeking to enhance the value of their stock.
Materials and Methods
The experimental protocols were adhered to in accordance with the National Regulations for the Use of Animals in Research in Vietnam. This includes compliance with The Law of Animal Husbandry of Vietnam, 2018 (Economica, 2018), and The Government Decree 32/2006/ND-CP (2006) on Management of Endangered, Precious, and Rare Species of Wild Plants and Animals. Notably, experiments on marine fish are exempt from the requirement for ethical approval as per these regulations. Throughout the duration of the experiment—including rearing, handling, and sampling—utmost care was taken to maintain fish in optimal conditions to ensure their well-being. All efforts were directed towards reducing stress and promoting the humane treatment of the fish used in this study.
The flowers (Fig. 1) of the leopard tree, wild sunflower, marigold, yellow bells and Wedelia chinensis were collected from various locations in Nha Trang city, Khanh Hoa Province. The fresh samples were dried at 60°C in 8 h in a convector dryer (Memmert UFE500, Gemini BV, Huizen, Netherlands). The dried materials (20 g) were then extracted using 200 mL of hexane as a solvent. The sample extraction was performed using a microwave oven (Sharp microwave 900 W, frequency 2,450 MHz, SHARP, Sakai, Japan) following the methodology described by Dang et al. (2018). The extract was filtered using filter paper, and the process was repeated three times until the filtrate became colorless. The pooled extract was collected, and the filtrates were concentrated using a rotary evaporator (IKA RV 10 control V, IKA, Königswinter, Germany). The crude carotenoid extracts were quantified using a UV-visible spectrophotometer (Directindustry, Marseille, France) to determine the carotenoid concentration in the sample.
The basal diet, formulated to contain 55% crude protein and 9% lipid, was developed in line with marine finfish feed requirements (Liu et al., 2014; Nankervis et al., 2022). Five carotenoid extracts were incorporated into the feed at a concentration of 0.25 g/kg feed: the control diet 0 g/kg (D-0), leopard tree (D-Lt), wild sunflower (D-Ws), marigold (D-Ma), yellow bells (D-Yb), and Wedelia chinensis (D-Wc) (Table 1). This protocol was adapted from the method described by Ebeneezar et al. (2020) with minor modifications. The ingredients for the feed (excluding the carotenoid extracts and vitamin mix) were accurately weighed according to the formula and thoroughly mixed homogeneously in a mixer. After mixing and adding water, the dough for the feed was steamed in a pressure cooker for 20 minutes. Following cooking and cooling, the dough with the incorporated carotenoid extracts and vitamin mix was extruded through an 800 µm mesh. The feed was then oven-dried at 60°C for 8h, subsequently crumbled, and sieved to form pellets. The feeds were stored in air-tight plastic containers and kept refrigerated until use.
Vitamin premix (mg/kg diet): Vitamin A, 1,000,000 IU; Vitamin D3, 300,000 IU; Vitamin C monophosphate, 10,000 mg; Pantothenic acid, 2,500 mg; Vitamin E, 2,000 mg; Vitamin B3, 2,000 mg; Vitamin K3, 500 mg; Vitamin B1, 500 mg; Vitamin B6, 500 mg; Vitamin B2, 320 mg; Folic acid, 200 mg; Biotin, 20 mg; Vitamin B12, 5 mg; Inositol, 10 mg; Choline chloride, 5 mg.
Mineral premix (mg/kg diet): Zn (ZnO), 4,750 mg; Mn (MnSO4.H2O), 1,900 mg; Mg (MgO), 1,050 mg; Co (CoCO3), 47.5 mg; Se (Na2SeO2), 47.5 mg; I (Ca(IO2)2 · H2O), 19 mg; P (CaHPO4 · 2H2O), 0.7%; Ca (CaHPO4.2H2O), 0.8%; Moisture, 10%; Ash, 2%; Ethoxyquin, 240 mg; Carrier (Dextrose), 86%. Provimi Vietnam, Bien Hoa city, Dong Nai province, Vietnam.
The golden trevally utilized in this study were artificially bred at the Duong De Marine Fish Hatchery, Aquaculture Institute, Nha Trang University, Vietnam. Selected fish were healthy, disease-free, and uniform in size, measuring an initial average of 2.80 ± 0.05 cm/fish in length and 0.46 ± 0.02 g/fish in weight. Prior to the experiments, fish were acclimatized to the conditions in the experimental tanks for one week. The setup included eighteen rectangular glass tanks (60 × 50 × 40 cm) lined with blue decal paper, each containing 100 liters of water and stocked with 30 fish. Tanks were continuously aerated to maintain oxygen saturation and received constant circulation of filtered seawater at a flow rate of 1.5 L/min. Weekly water changes replaced one-third of the volume. Diets supplemented with floral-source carotenoids at 0.25 g/kg of feed were administered, following the experimental design (three replicates per treatment), except for the control group. Feedings occurred four times daily at 7h30, 10h30, 13h30, and 16h30. Water quality parameters were controlled at a temperature of 28.5 ± 0.72°C, salinity of 33.4 ± 0.98‰, pH of 7.91 ± 0.19, dissolved oxygen of 5.5 ± 0.21 mg/L, and unionized ammonia nitrogen of 0.05 ± 0.01 mg/L. The feeding trial conformed to the natural light-dark cycle over the 56-day duration.
Growth performance and feed utilization were assessed at the end of the experimental period. Following a 24-hour fasting protocol, specimens were anesthetized using 0.05% ethylene glycol monophenyl ether, enabling the evaluation of growth indices, physiological health, and pigmentation. Parameters measured incorporated final total length (L2), final body weight (W2), specific growth rates for length (SGRL) and weight (SGRW), coefficients of variation for length (CVL) and weight (CVW), condition factor (K), survival rate (SR), as well as feed utilization parameters including feed intake (FI), feed conversion ratio (FCR), protein efficiency ratio (PER), and lipid efficiency ratio (LER). The calculations for these metrics are detailed as follows:
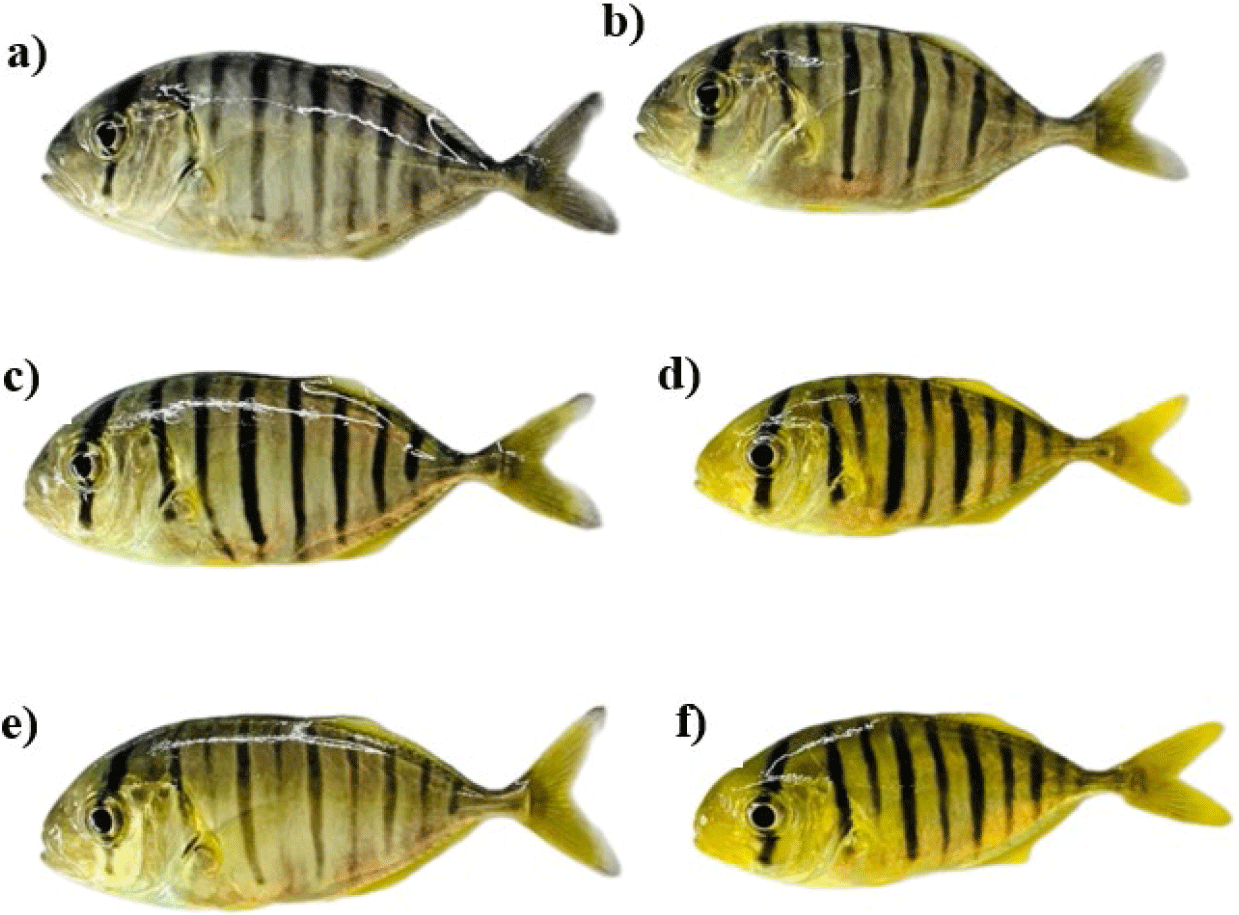
The coloration of the fish skin (Fig. 2) was measured bilaterally on the body of the fish, at the midpoint between the bases of the pectoral fins and the pelvic fins. Triplicate measurements were taken at each position using a chroma meter CR-400 (Konica Minolta, Osaka, Japan). Assessment of skin pigmentation was performed by reflectance spectroscopy. The color parameters were CIE L* for lightness ranging from 0–100 for black to white, CIE a* for red/green, and CIE b* for yellow/blue. For example, CIE L* of 100 indicates absolute brightness, whereas a value of 0 designates absolute darkness. The (+) higher positive ‘CIE a*’ and ‘CIE b*’ show a higher level of redness and yellow-orangeness, while (–) negative values of ‘CIE a*’ and ‘CIE b*’ express green and blue, respectively.
The proximate compositions of the experimental diets were analyzed according to the standard methods outlined in AOAC (2005). The samples were dried to a constant weight at 105°C in an oven to determine moisture content. Crude protein (N × 6.25) was analyzed using the Kjeldahl method. Crude lipid was determined by ether extraction using the Soxhlet system, and ash content was measured after combustion at 550°C for 24 h (Table 1).
Total carotenoid content in the feed, skin, muscle, and the whole body of the fish was analyzed using a UV-visible spectrophotometer following the method described by García-Romero et al. (2014) and Ramamoorthy et al. (2010) with minor modifications. First, samples of skin (1 g) were collected from both sides of the fish, while muscle (1 g), whole body (1.0 g), and feed (1.0 g) were also prepared. The samples were ground in acetone (20 mL) containing 1.5 g of anhydrous sodium sulfate with a homogenizer (model T10, Ultra-turrax®, IKA). The samples were then filtered using filter paper, and the process was repeated three times until the filtrate became colorless. After that, the filtrates were centrifuged at 13,200×g at a temperature of 4°C for 15 min. Lastly, absorption was measured at 485 nm using a spectrophotometer (Biochrom, Cambridge, UK). The results were expressed as micrograms per gram (µg/g) and calculated using the following equation:
where: A is the absorbance; V is the total volume of the extract (mL); D is the dilution ratio; W is the weight of the sample (g); and an extinction coefficient (E) of 2,100.
All experiments were performed in triplicate. The percentage data were arcsine-transformed before statistical analysis to meet the assumptions of ANOVA. The homogeneity of variances and normality of the data were also checked and confirmed. The transformed data were expressed as mean ± SE (n = 3), while the environmental parameters were presented as mean ± SD. One-way ANOVA and Duncan’s post hoc test were used (SPSS Statistical Software, Version 22.0, IBM, Chicago, IL, USA) to analyze the differences between the samples. Differences between the mean levels of carotenoids, color expression, and growth parameters of fish in different experiments were considered statistically significant if they had a p-value of < 0.05.
Results
After a 56-day trial period, the growth performance indicators for length and weight in golden trevally fish fed with pigment-enhanced flower supplements demonstrated significant improvements compared to the control group (p < 0.05; Table 2). Specifically, fish consuming feed enriched with marigold flower extract exhibited the highest growth performance for length (L2, SGRL) and weight (W2, SGRW), with statistically significant differences when compared to groups receiving other flower extracts (p < 0.05). Remarkably, there was no significant difference in these growth performance indicators between the group fed with marigold extract and the group fed with wild sunflower extract (p > 0.05). The indicators for the coefficient of variation in length and weight also showed better results in the group fed with flower pigment supplements from marigold, wild sunflower, and yellow bells compared to the control group, indicating a more uniform size among the fish (p < 0.05). However, no differences were observed in the remaining indicators, including the condition factor and SR, between the groups supplemented with flower pigments and the control group (p > 0.05).
The inclusion of flower pigments in the diet also affected feed conversion efficiency in golden trevally fish (Table 3). The group supplemented with flower pigments consumed less food compared to the control group, but the indicators for feed conversion efficiency were significantly better (p < 0.05). Specifically, the FCR, protein utilization efficiency, and lipid utilization efficiency were best in the group fed with marigold flower extract, showing significant differences from the control group and the group supplemented with Wedelia chinensis (p < 0.05). However, there was no significant difference in feed conversion efficiency between the groups supplemented with marigold extract and those supplemented with other types of flowers, including leopard tree, wild sunflower, and yellow bells (p > 0.05). These results have demonstrated the role of flower pigment supplements in promoting feed conversion efficiency in the diet of golden trevally fish, particularly with marigold flowers.
The proximate composition of golden trevally was assessed at the end of the feeding trial and is detailed in Table 4. The average composition was as follows: moisture content at 70.6 ± 0.95%, crude protein at 15.1 ± 0.19%, lipids at 5.18 ± 0.11%, and ash at 3.04 ± 0.44%. The analysis revealed no statistically significant differences in the body composition of golden trevally when comparing the groups fed with various floral extracts over the 56-day period.
The spectrophotometric analysis of skin pigmentation in golden trevally fed with different natural carotenoid sources for a period of 56 days is reported in Fig. 2 and Table 5. Diets supplemented with carotenoids derived from five different flower extracts resulted in a significant enhancement of yellow pigmentation (CIE b*) in golden trevally when compared to the control group (p < 0.05). The CIE a* were negative (−) across all experimental groups at the end of the experiments, indicating a greenish hue. The highest CIE b* (18.8 ± 0.42) was recorded for the D-Ma group, followed by the D-Ws group (17.2 ± 0.85). The D-Yb group exhibited the lowest CIE b* (13.2 ± 0.65). However, no significant differences were observed in the lightness (CIE L*) of the fish across all dietary groups.
The total carotenoid content in five flower species is presented in Table 6. The marigold extract exhibited the highest carotenoid content (229.59 ± 9.79 µg/g), followed by wild sunflower (182.62 ± µg/g) and Wedelia chinensis (117.82 ± 8.08 µg/g) extracts. Lower carotenoid concentrations were observed in the extracts of leopard tree (75.54 ± 3.34 µg/g) and yellow bells (63.17 ± 3.36 µg/g), respectively.
| The carotenoid content (µg/g) | D-Lt | D-Ws | D-Ma | D-Yb | D-Wc |
|---|---|---|---|---|---|
| 75.54 ± 3.34a | 182.62 ± 4.46c | 229.59 ± 9.79d | 63.17 ± 3.36a | 117.82 ± 8.08b |
Data from Table 7 indicate that carotenoid deposition in the skin of golden trevally was significantly higher than in the muscle. Furthermore, diets supplemented with various flower extracts led to significant variances in carotenoid accumulation within the fish (p < 0.05). There was also a notable correlation between the yellow pigmentation (CIE b*) and the carotenoid content in the fish skin (Tables 5 and 7). The highest carotenoid concentration in the skin (71.2 ± 2.9 µg/g) was recorded in fish fed with a diet containing marigold extract (D-Ma sample), which was over seven times higher than that in the control group (10.7 ± 0.43 µg/g). High carotenoid levels were also detected in the D-Ws (56.3 ± 1.69 µg/g) and D-Wc samples (46.6 ± 1.94 µg/g). The distribution pattern of carotenoid accumulation in fish muscle and the whole fish was akin to that observed in fish skin with various carotenoid sources. The most elevated carotenoid concentrations (muscle: 0.69 ± 0.05 µg/g; whole fish: 4.04 ± 0.06 µg/g) were found in fish fed diets supplemented with marigold flower extract.
Discussion
Carotenoids are divided into two main groups based on their structures: carotenes and xanthophylls. Carotenes are hydrocarbons, including β-carotene, α-carotene and lycopene, while xanthophylls contain oxygen atoms in their molecules, examples being lutein, zeaxanthin, astaxanthin and canthaxanthin (Saini et al., 2015). Carotenoids accumulated in flowers are responsible for color diversity (yellow, orange, and red colors) that attracts pollinators. All flower species in our study, including the leopard tree, wild sunflower, marigold, yellow bells and Wedelia chinensis exhibit a yellow hue. Research has revealed that the majority of carotenoids in flower petals are yellow xanthophyll, such as lutein, zeaxanthin, cryptoxanthin, violaxanthin, antheraxanthin, and neoxanthin (Ohmiya, 2011). In marigold (T. erecta), the range of petal color varies from pale yellow to orange, attributable to different levels of the yellow xanthophyll (primarily lutein), while differences in carotenoid composition have been shown to lead to color differences observed in the yellow and orange petals of calendula (Ohmiya, 2013). On the other hand, flavonoids/anthocyanins and carotenoids often coexist in the same plant parts, and their combination further enhances color variety (Tanaka et al., 2008).
The compositional analysis and application of marigold flowers to enhance growth and coloration in various fish species have been well-documented in previous studies (Ohmiya, 2011, 2013; Ratananikom et al., 2021). Conversely, details regarding the utilization of other yellow-pigmented flower species, including the leopard tree, wild sunflower, yellow bells, and Wedelia chinensis, in aquaculture remain scarce. The carotenoid levels measured in the five flower species (as indicated in Table 6) reveal that these two species possess higher concentrations relative to the others. Marigolds and wild sunflowers exhibit more intense yellow hues compared to the rest flowers through the results measured by a chroma meter CR-400 (data not shown), and our findings are in agreement with those of previous studies that the deeper the yellow color of the flower was, the higher the carotenoids content in the flower had (Ratananikom et al., 2021).
The role of carotenoids in enhancing fish growth remains a subject of debate, with studies indicating both beneficial and negligible outcomes. Fish growth in aquaculture is acknowledged to be influenced by a multitude of factors, including nutritional composition, feeding regimes, and environmental conditions. Our study indicates that carotenoids derived from the extracts of the five flowers significantly enhance growth parameters in fish. It is believed that carotenoids play a role in promoting feed efficiency and fish growth possibly due to the simultaneous effects of many factors. Carotenoids have been shown to enhance nutrient utilization efficiency by regulating intermediary metabolism through controlling the activity of digestive enzymes (Amar et al., 2002). The indirect effects of carotenoids when added to the diet include antioxidants, stress reduction, and regulation of gene expression of physiological processes (nutrition metabolism, growth regulation, immunity), endocrine regulation (growth hormones, IGFs) also contributes to optimizing energy use for growth as well as maintaining fish health (Elbahnaswy & Elshopakey, 2024). Carotenoids are thought to promote the activity of intestinal microbiota to help break down indigestible nutritional components (e.g. fat), and optimize this energy source for growth of fish (James et al., 2006). The addition of carotenoids to the feeding regimen had a positive effect on the length and thickness of the villi and the thickness of the intestinal wall – factors that are believed to contribute to improving the ability of fish to absorb and utilize nutrients from food (Zhao et al., 2022). These results are corroborated by numerous studies, which have reported marked improvements in specific growth rates and weight gain in sword-tail fish (Xiphophorus helleri) with diets including China rose, marigold, and carrot (Rana et al., 2023); in Barilius bendelisis with spirulina and marigold (T. erecta) (Jha et al., 2012); in seabass (Dicentrarchus labrax) with synthetic astaxanthin, marigold meal, and crab waste meal (Goda et al., 2018).
Carotenoid pigments may be sequestered either directly within the chromatophore cells of fish or undergo transformation through cellular metabolism (Sathyaruban et al., 2021). Fish are capable of both reducing and oxidizing dietary carotenoids, which facilitates their transformation from one form to another. The deposition of carotenoids in different fish species occurs in various tissues, such as integuments, gonads, and muscles (Singh et al., 2021; Wassef et al., 2010). The coloration of fish is the result of several internal and external factors: chromatophores, species, genetics, developmental stages, metabolic capacity, physiological condition, and health status, along with nutritional supplementation, environmental factors, and social interaction (Luo et al., 2021; Sathyaruban et al., 2021; Wassef et al., 2010). Chromatophores, cells containing color pigments in fish skin, utilize carotenoids to produce yellow (xanthophylls), red and orange (carotenoids), and brown and black (melanin) pigments (Yeşilayer et al., 2020). Lutein has been identified as a common carotenoid in both freshwater and marine species (Gupta et al., 2007), while zeaxanthin is known to be metabolically converted from astaxanthin by some fish species (Maoka, 2011).
In our findings, the CIE a* were negative for all groups. However, there was a significant difference in the CIE a* of golden trevally when fed with dietary marigold flower extract compared to other extracts and the control. The negative CIE a*, and the statistically significant difference in the fish coloration when supplemented with carotenoid extract compared to the control were also observed in electric yellow cichlid (Labidochromis caeruleus) supplemented with 150 µg/g marigold (T. erecta) extracts (Yeşilayer et al., 2020); in Blue Streak Hap (Labidochromis caeruleus) and Pindani (Pseudotropheus socolofi) Fry Cichlidae with 4% marigold flower meal (T. erecta) (Cavdav et al., 2020). The highest CIE b* of fish was achieved with marigold extract; and the differences in CIE b* among all extracts and the control sample were found to be significant (p < 0.05). It was evident that the carotenoid extracts from five flower species enhanced the yellow pigmentation of fish. However, the carotenoid composition in different flowers resulted in varying degrees of yellow coloration on the golden trevally’s skin. The findings were in line with those reported by Yeşilayer et al. (2020) in electric yellow cichlid (Labidochromis caeruleus) fed with 150 µg/g marigold (T. erecta) extracts, by Jorjani et al. (2019) in the blue gourami (Trichogaster trichopterus) with marigold content (Calendula officinalis) in the diet. No significant differences were noted in the lightness (CIE L*) across all groups supplemented with different carotenoid sources, and the findings were similar to reports from previous studies (Cavdar et al., 2020; Yeşilayer et al., 2020).
Carotenoids extracted from marigold flower (T. erecta) are predominantly composed of lutein (approximately 80%), zeaxanthin, and β-cryptoxanthin (Rodrigues et al., 2019; y Juan et al., 2013). Therefore, it is hypothesized that these three pigments, particularly lutein, significantly influence the yellow pigmentation of golden trevally skin. This hypothesis aligns with the findings of Sánchez et al. (2020), who reported that the yellow coloration of rainbow trout (Oncorhynchus mykiss) is affected by the composition and level of carotenoids obtained from bee pollen. A similar enhancement in the yellow color of electric yellow cichlid (Labidochromis caeruleus) was observed after the addition of marigold (T. erecta) extracts (Yeşilayer et al., 2020). However, this color enhancement was not replicated in the blue gourami (T. trichopterus) with feed supplemented with marigold powder (Calendula officinalis) (Jorjani et al., 2019).
Our study determined that total carotenoid content and skin color intensity are correlated with the level of carotenoids accumulated in fish tissue. The results echo previous studies that have shown natural carotenoids to be crucial in color expression in several fish species. Ho et al. (2013) reported that an increase in dietary carotenoid concentration (esterified astaxanthin) corresponded to a rise in total skin carotenoid concentration (clown anemonefish, Amphiprion ocellaris). Total carotenoid content in fish (European seabass, D. labrax) significantly increased with increasing dietary carotenoid levels (marigold flower and crab waste meal), irrespective of the carotenoid source (Goda et al., 2018). The highest total carotenoid concentration in muscle tissue was observed in Koi carp, Cyprinus carpio L., fed a diet containing 180 ppm of marigold oleoresin (Swian et al., 2014); in goldfish (Carassius auratus L.) with marigold meal (T. erecta) (200 mg/kg feed) (y Juan et al., 2013).
Conclusion
The results suggest that two of five flower species are effective dietary carotenoid sources for this species over a rearing period of 56 days. The observed high values of color expression (CIE b*) and total carotenoid content in the fish skin were recorded at 18.8 ± 0.42 and 71.2 ± 2.9 µg/g, respectively. The utilization of natural carotenoid sources from agricultural products proves to be more cost-effective, sustainable, and healthier in aquaculture. The outcomes of our study highlight marigold and wild sunflower as valuable natural carotenoid sources, crucial for enhancing both red pigmentation and growth performance in farmed golden trevally.
