Introduction
To contribute to reducing the pressure of fishing as well as protecting some economically valuable fish species that are endangered by extinction, the creation of artificial seeds for aquaculture is an issue of concern of interest to aquarists. The selection of broodstock and determining the matured stage of the ovaries are crucial factors that aftect the success of fingerling production in hatchery conditions. Surveys and sex determinations are still invasive and do not meet animal welfare standards.
Currently, the application of non-invasive techniques in breeding to ensure animal welfare has received special interest by farmers in many countries. Twenty females of the African catfish (Clarias gariepinus) were subjected to tomography using a SIUI CTS-5000 ultrasound machine equipped with a 3.5/5.0 Mhz inverter-type curve probe. Ultrasound was used as a decisive diagnostic tool for breeding (Achionye-Nzeh & Jimoh, 2010). Different types of abnormality of the gonads in this species are identified by ultrasound images. These anomalies cannot be detected by a simplified and conventional observation - only based on the secondary sex characteristics. The accurate female anatomy shows that the female has a single ovary instead of 2 ovaries like other fishes. This single-ovary structure has a great impact on absolute fertility as this fish is subjected to artificial breeding by using hormonally induced ovulation. In addition, the study results demonstrate the practical usefulness of ultrasound as a rapid, non-invasive, and real-time maturation diagnostic method for flounder juveniles (Matsubara et al., 1999). Ultrasound imaging techniques for the reproduction in many high-value aquatic animals are further mentioned by various authors such as Guitreau et al. (2012); Hliwa et al. (2014); Mattson (1991); Næve et al. (2018, 2019); and Novelo & Tiersch (2016). These studies showed that ultrasound is an effective method to assess the fertility of broodstock by allowing the hatchery owners to detect abnormalities in fish gonads. However, no scientific research has been published on this issue in Vietnam so far. This study aims to give the ultrasonography methods in studing catfish gonad development.
Methodology
Mindray ultrasound machine with Convex Probe with frequency of 5 MHz was used in this study based on the research result of Hoa et al. (2024).
Twenty striped catfish (Pangasianodon hypophthalmus) were purchased from the hatchery, then transported to the fish laboratory of the Faculty of Fisheries for ultrasonic determination. These fish are prepared to adapt to the 1,000 L composite tank environment. Anesthetize the fish with AQUI-S® anesthetic (Bayer, Vietnam), containing 50% Iso-eugenol extracted from plants, a dose of 25 mL for 1 m–3 of water for 3 minutes. After the fish shows signs of anesthesia (loss of balance, straightened fins, stops swimming, loss of reflexes to stimuli, slow respiration), take the fish out, weigh the fish’s weight, and record the data. Place the fish on an ultrasonic mattress to make it more comfortable and easier to hold. Use Sky ultrasound gel (Malaysia) as the ultrasound transmission medium. Ultrasound images were determined in 2 groups: A (3–5 kg) and B (> 5 kg).
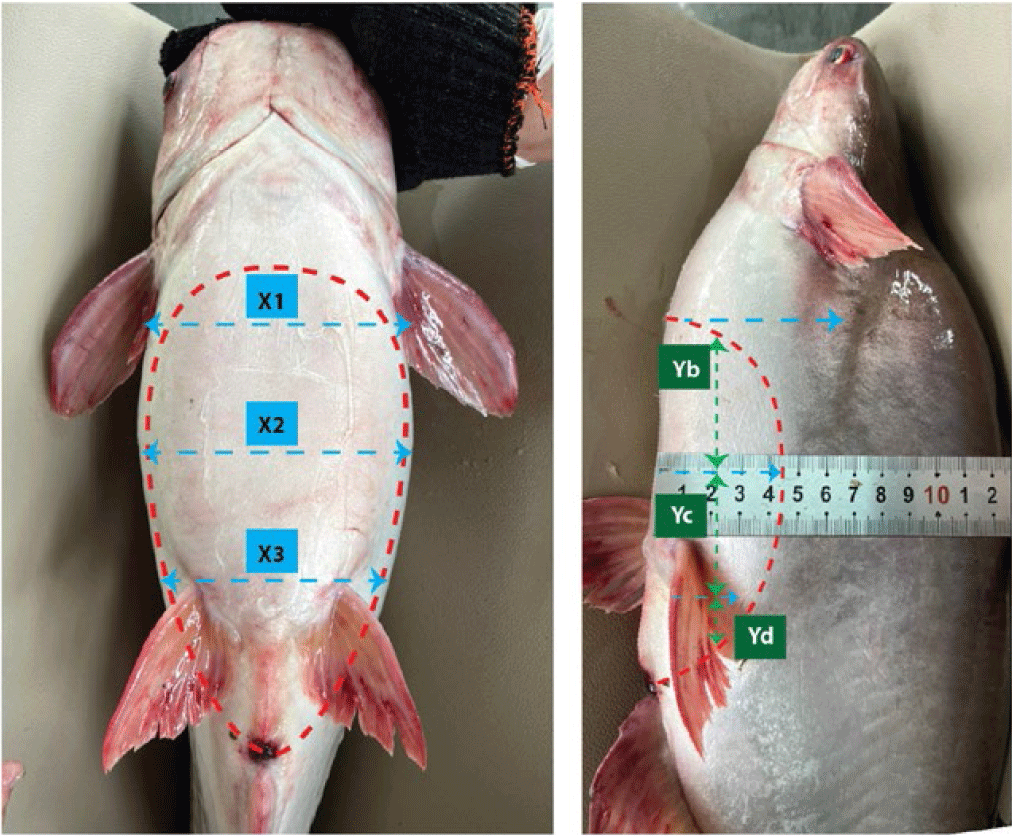
Read the ultrasound results by moving the probe horizontally and vertically along the body of the catfish, at the 6 positions determined as in Fig. 1 until the image on the ultrasound machine screen is clear. Mostly, displayed as points with brightness or gray scale, specifically: ultrasound images of male pangasius gonads are large or small black, representing large or small sperm chambers; Ultrasound images of female pangasius gonads are large or small white, showing large or small ovaries.
Monitoring criteria: the image on the screen representing the gonadal structure of male and female, the brightness of the image, and the reflection levels presented on the ultrasound images.
Results and Discussions
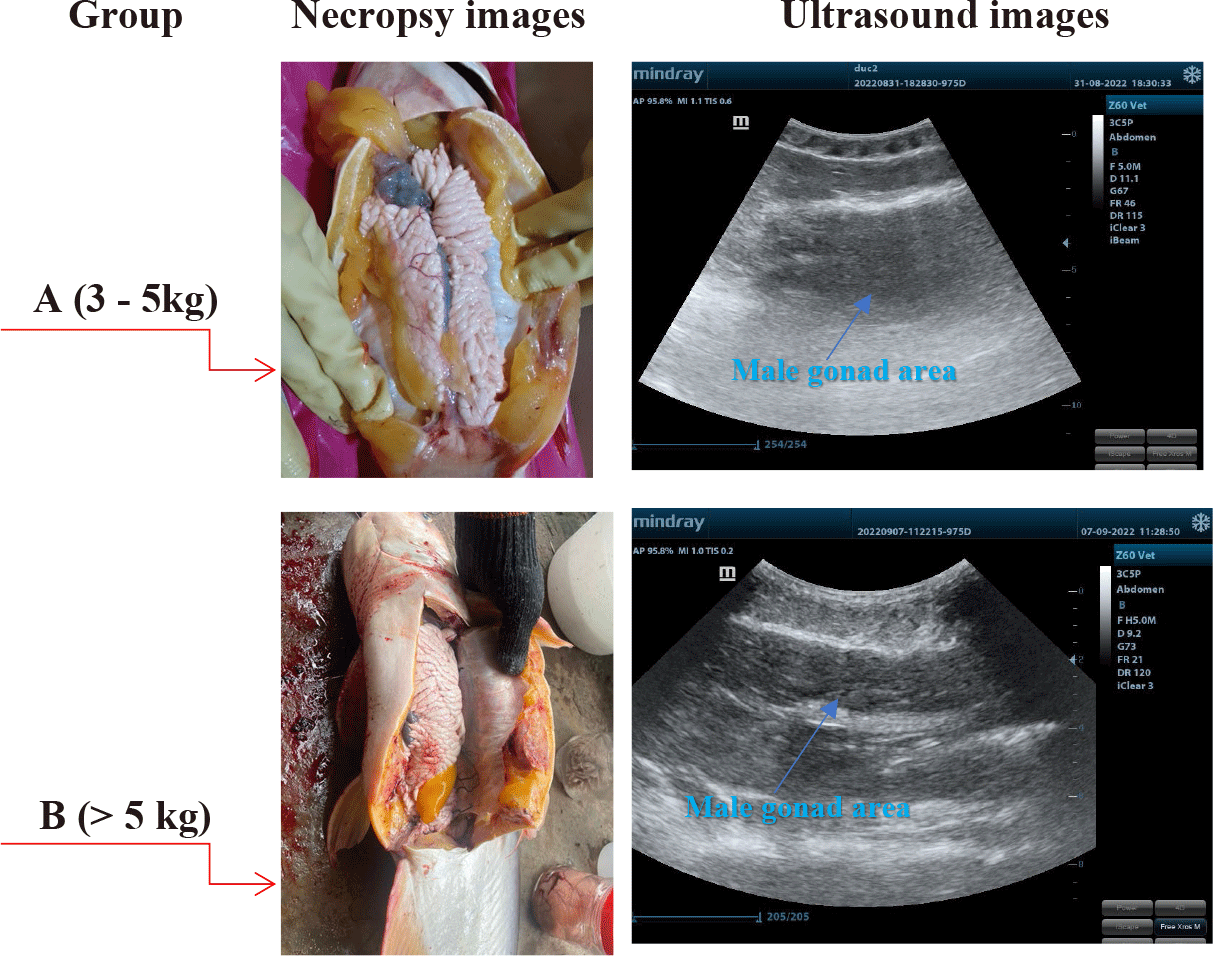
A Convex probe at 5 MHz was used to perform the ultrasound examination of the male gonads in striped catfish. Ultrasound images are presented in Fig. 2.
The results indicated that the male gonads of striped catfishes less than 5 kg, identified that immature ones by necropsy examinations were not distinguished from other visceral organs by using ultrasonic technique. This result was similar to the study of Kucharczyk et al. (2016) in European eels (Anguilla anguilla). The authors used a SonoSite USG (M-Turbo, 5 MHz) ultrasound machine and pointed out that ultrasound was helpful for European eels’ sex determination and highly accurate biopsy. This was a non-invasive method that allowed us to quickly determine the gonad’s maturity. A hand-held ultrasound machine could be used either in both laboratory or on-spot. Novelo also reported that the ease of differentiating between male and female fish decreased for smaller-sized gonads.
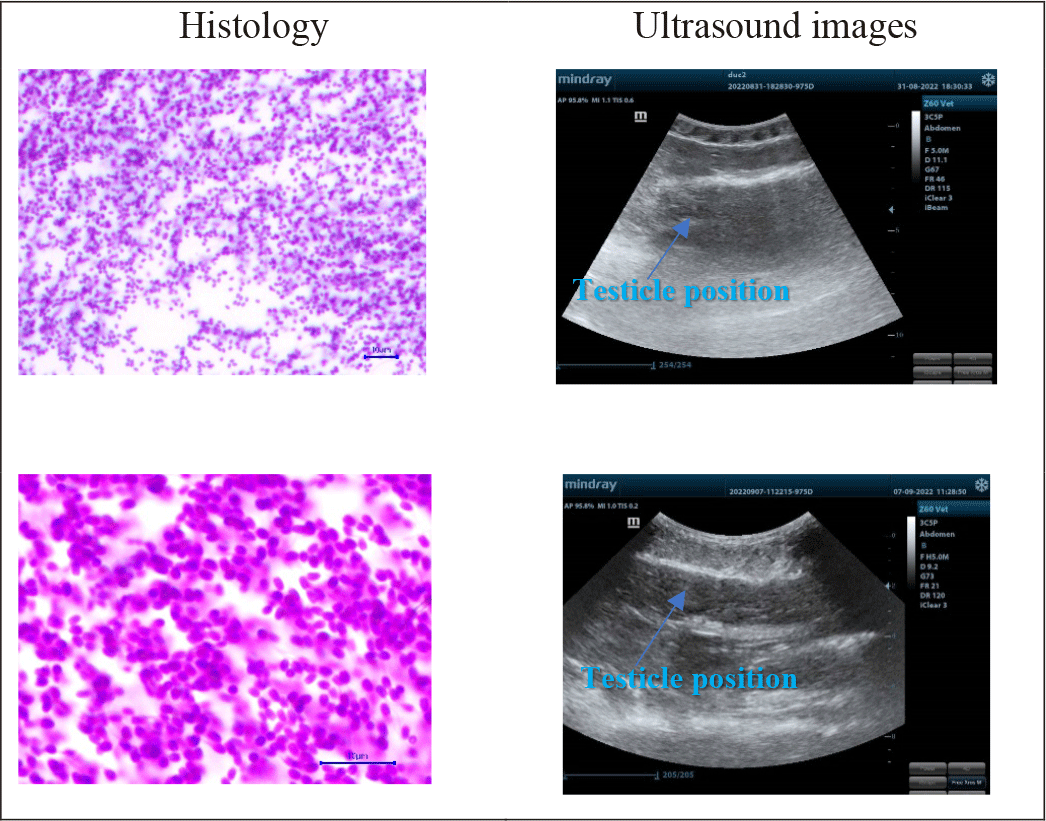
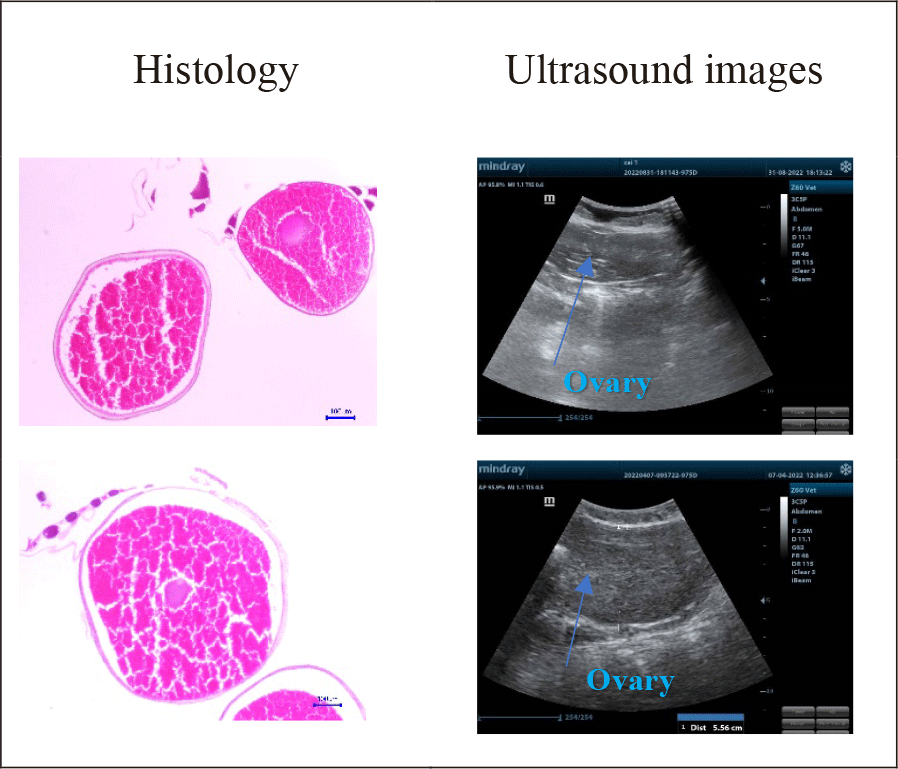
Maturity stages and testicle sizes in the catfish less than 5 kg via the combination of ultrasound and histology were demonstrated in Fig. 3.
The results from Fig. 3 illustrated that male gonads did not present transparently on ultrasound images. It resulted from the testicle having a smaller size than the ovary, so the received - images were less clear. When the fish were in further development stages, particularly when the male gonads were at stage III, the ultrasound images were more defined, and testicle histological slides were larger and more remarked. Histological examination indicated that:
Male samples in the group less than 5 kg were in stage II: histological examinations showed that the amount of sperm clutches was higher than that in the previous stages. There were a high number of spermatogonia, a few primary spermatocytes, and spermatozoon. Cell’ sizes were still large in each ovarian lobule. Spermatids had smaller nuclei and darker chromatins. However, the ultrasound images were not clear and blurred, spermatid - clutches appeared in white; the gonads were immature and could not be distinguished from other visceral organs.
Male samples in group higher than 5 kg, stage III: Spermatid and spermatozoon populations increased gradually, and spermatozoon clutches were much more than other cells in histological slides. It also showed that it was difficult to detect sperm on ultrasound images. Although it was presented in black and white points, the testicles still could be differentiated from other organs.
The tops of ovaries were estimated at Ya, the middles were estimated at Yb and the ends were estimated at Yc in Striped catfish.
Ultrasound images determined on 2 groups: A (3–5 kg) and B (> 5 kg) were indicated in Fig. 4.
Ultrasonography was performed in 10 pcs/group and the results were presented in Table 1.
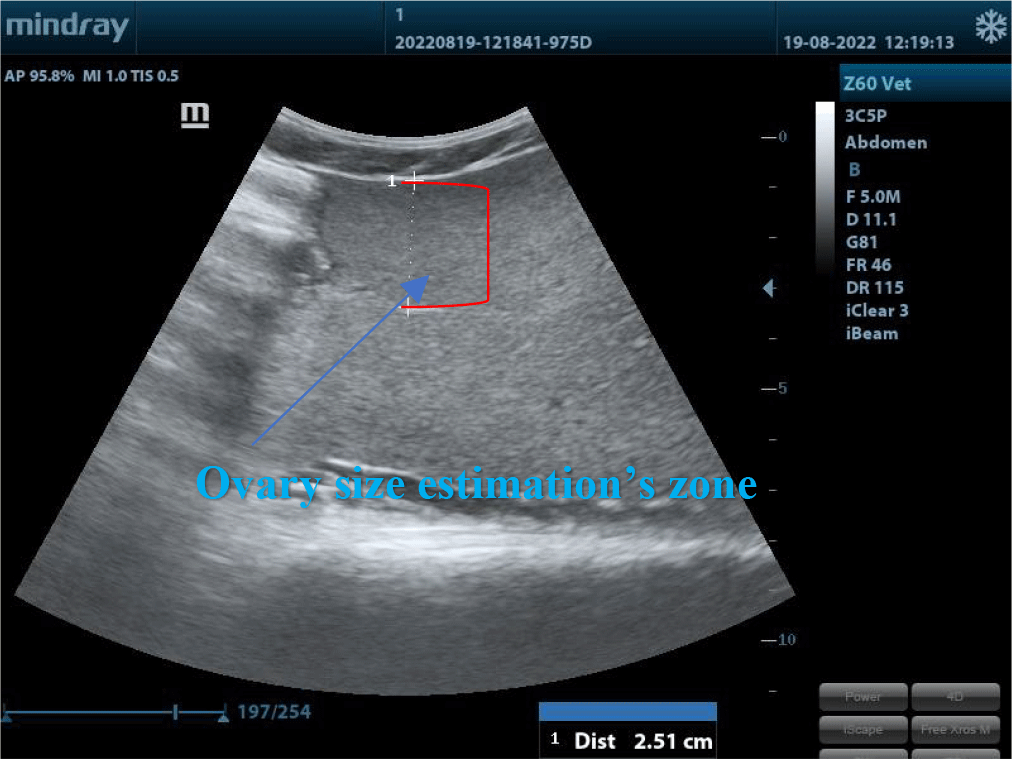
Table 1 showed that ovarian diameters were different between the two groups at the different ovarian sites and fish body weights. It was explained that when the body weight reached over 5 kg, the ovary size mostly rose slightly. At this time, only oocytes’ diameters could not be estimated as these fishes did not grow up enough and were still immature. This result was tallied with the report of Moghim et al. (2002) about the sex and maturity determination in Acipenser stellatus by ultrasound. The authors used Model 4091 5.5/7.5 MHz 64E VET and concluded that the accuracy in sex and maturity age determination was up to 97.2%.
Striped catfish did not have any accessory gonads (secondary gonads) leading to the difficulty in differentiating males and females when they are immature. At the mature age, testicles (in males) and ovaries (in females) developed remarkably. In the further stage, the ovary was larger and the oocytes changed into yellow; testicles in males had branched morphology and changed from pink to milky white.
Maturity levels and ovarian size assessments by ultrasound examination, microscope observation, and histology were illustrated in Fig. 5.
The changes in the size of the oocytes on ultrasound images according to the development stages of female gonads are demonstrated in Fig. 5. The female gonads in group A were all in stage II. The cytoplasm of ovarian follicles was developing, weak base-staining, and had some lipid droplets. The eggshell was thin and contained numerous vitellus presenting in small black-white and smooth dots on the ultrasound images. The diameter was very small. The female gonads in group B were all in stage IV. The ratio of nucleus and cytoplasm decreased. There was an accumulation of lipids and vitellus. The thickness of egg membranes was thicker than in previous stages and showcased in small white and black dots. Egg diameters estimated by ultrasound ranged from 1,6 mm to 1,8 mm. In this stage, artificial reproduction could result in great reproductive performance.
Ultrasound images in both groups A and B had significant differences. In group B, the oocyte’s dimensions on the ultrasound images were twice bigger than group A were. Oocytes were in black and white spots, which resembled Martin-Robichaud & Rommens (2001) research in 4-year Atlantic turbot (54.8 ± 71.2 cm; 2.3 ± 5.2 kg) with frequency set at 5 Mhz and 110 ± 120 mm in depth.
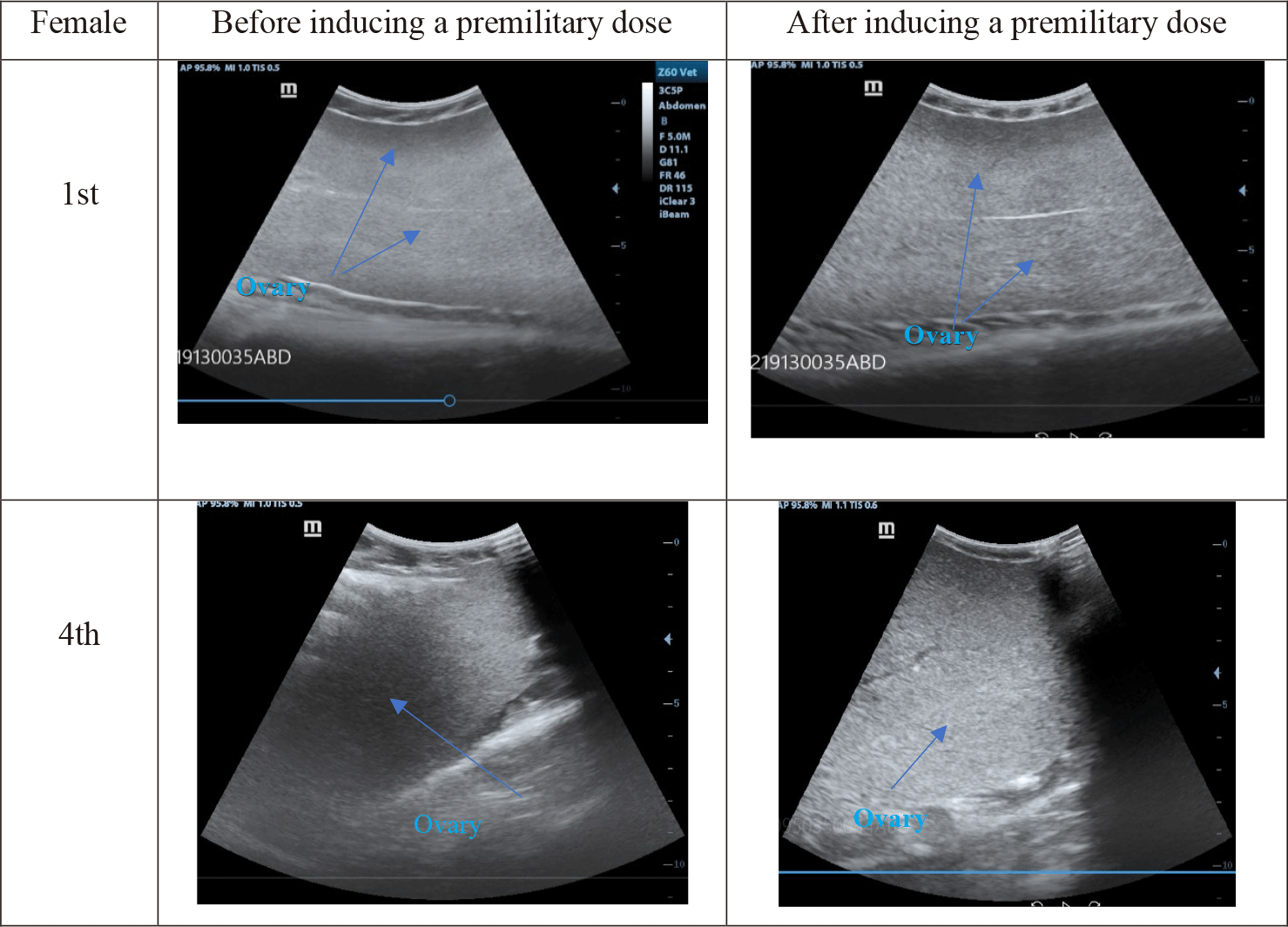
Dimension modifications of ovaries and oocytes in 10 striped catfish before and after hormonal induction were expressed in Fig. 6 (blue arrows). Ultrasound images of 10 fish were homogenous and were described typically in the 1st and 4th individuals.
Before the premilitary dose, eggs displayed small, smooth, black dots on the ultrasound images (Fig. 6). After the premilitary dose, the eggs became bigger and had brighter dots. After the definitive dose, oocytes’ diameters could be measured more easily.
Egg sizes were measured by ultrasound machine during hormonal induction shown in Table 2.
| Values | Before the premilitary dose (mm) | Before the definitive dose (mm) | 12h after the definitive dose (mm) |
|---|---|---|---|
| Mean | 0.53a ± 0.01 | 1.11b ± 0.02 | 1.56c ± 0.02 |
Before the premilitary dose, egg sizes estimated by ultrasound were too small (0.53 mm). After the definitive dose (12 h after injection), egg size increased two times. 12 h after the definitive dose, egg diameters raised twice and peaked at 1,8 mm in the 4th individual. The eggs developed into stage IV and the abdomen was distended. It was the best time for spawning. This result also similar to the research of Duong & Ta (2023). They also found that the size of striped catfish egg ranged from 1.014–1.024 µm.
Conclusion
The development of striped catfish gonads were identified by ultrasound images, especcially this utility showed the good effects when scanning the matured ones. The male immature gonads of striped catfishes were not distinguished from other visceral organs by using an ultrasonic technique. The male gonads were at stage III, and the ultrasound images were more defined, remarked. The ovary diameter and egg size could be measured by ultrasonography. The ultrasonic technique made breeding production more efficient and meets animal welfare goals. This technique will be applied to fish hatcheries.