Introduction
The whiteleg shrimp, Litopenaeus vannamei, is a widely cultured species of high economic value. The whiteleg shrimp is very popular because of its high-quality meat, good taste, good nutritional content, and easy cooking (Li et al., 2021). Whiteleg shrimp is also one of the most successful cultured species in aquaculture, accounting for up to 80% of shrimp production (Rosilan et al., 2023). Whiteleg shrimp has been cultivated with intensive to super-intensive technology (Mustafa et al., 2023).
However, whiteleg shrimp have several challenges from various diseases, including Acute Hepatopancreatic Necrosis Disease (AHPND), a bacterial disease also known as Early Mortality Syndrome (EMS) (Rosilan et al., 2023). AHPND has caused significant economic losses for shrimp (Ha et al., 2023). Vibrio parahaemolyticus is a well-known causative agent of AHPND in penaeid shrimp farming worldwide (Velázquez-Lizárraga et al., 2019). V. parahaemolyticus, a Gram-negative bacterium that lives in marine and estuary environments, becomes pathogenic in shrimp at concentrations greater than 104 CFUs/mL (Soto-Rodriguez et al., 2022).
The use of immunostimulants to enhance immune responses is one of the important steps that can be taken to prevent disease in shrimp aquaculture (Kumar et al., 2022). Immunostimulants are substances that can activate or enhance the activity of the immune system, used to help the body’s immune response in combating infections (Di Sotto et al., 2020). Immunostimulants are considered an attractive and promising agent for preventing diseases in fish and shellfish. Increasing the ability of shrimp bodies to fight disease by administering immunostimulants can be indicated by several parameters, including prophenoloxidase (proPO), total hemocyte counts (THC), and differential hemocyte counts (DHC). When selecting these therapeutic agents, it’s important to think about the safety of consumers, the environment, and the shrimp.
Immunostimulants derived from plants are organic, free from side effects, environmentally friendly, and safe to use to improve the immune system of shrimp (Kumar et al., 2022). C. odorata, also known as Siam weed (Eze & Jayeoye, 2021) is a rapidly growing perennial herb that can grow up to 2.5 m (100 inches) tall in open areas and up to 10 m (33 feet) tall in shady areas where it behaves as a creeper, growing on other vegetation. When crushed, the plant is hairy and glandular; the leaves give off a pungent, aromatic odor. Some of the benefits of C. odorata are that it can improve soil fertility, crop production, protection, nutrition, and ethnopharmacology (Mugwedi, 2020). C. odorata has been reported to have antibacterial, anti-inflammatory, antioxidant, anthelminthic, antifungal, cytotoxic, anticonvulsant, antiprotozoal, and wound-healing properties (Mugwedi, 2020). This antioxidant and antibacterial property could help protect aquatic animals from oxidative stress and bacterial infection caused by environmental factors (Eze & Jayeoye, 2021).
Different plant seasons or life cycle stages can give different results in analyzing bioactive compounds. The leaves of C. odorata contain phytochemicals such as tannins, alkaloids, flavonoids, saponins, terpenoids and other phenolic compounds, which produce a physiological action in the body (Rasyid et al., 2020; Zahara, 2019). The presence of these components in C. odorata leaf extract has shown that it can be a source of immunostimulant to improve shrimp antibacterial activity. Various factors, including the environmental condition, can influence shrimp’s growth and immune response. In the earlier study, in controlled testing, C. odorata leaf flour components (CO) enhanced immune responses and Survival Rate (SR) in tiger shrimp infected with V. harveyi (Harlina et al., 2019). However, the impact of CO use in improving the growth and health of farm-farmed whiteleg shrimp L. vannamei is still an area of research that has not been revealed. Thus, this study examines the effectiveness of CO at the optimal dose to increase the growth performance, non-specific immune activity, and resistance against V. parahaemolyticus of whiteleg shrimp L. vannamei culture in the pond.
Materials and Methods
The old leaves of C. odorata were collected in the rainy season around a pond in Marana Village, Maros, Indonesia. followed by cleaning under running water and then cut into small cube-like pieces. The resulting leaf pieces were then dried using a drying cabinet at 40°C. The dried leaves were then turned into flour with the help of a kitchen blender. The leaf flour was then divided into two parts; some were used for phytochemical tests, while the other was mixed into feed as part of experiments.
Approximately 10 g of C. odorata leaf flour was extracted with 200 mL of methanol (MeOH) while stirring using a shaker for 24 hours at room temperature. The MeOH extract was filtered using Whatman No.4 filter paper. The MeOH extract was then concentrated using a rotary evaporator set at 37°C to obtain concentrated MeOH extract. Phytochemical tests were conducted to confirm the uniformity of compound content in C. odorata leaves used in this study with compound content in C. odorata leaves that had been investigated previously (Harlina et al., 2013). The components present were identified through the observation of color changes or the formation of visually visible deposits.
The flavonoid analysis involved combining 1–2 mL of warm 50% MeOH with 1 mL of MeOH extract, then introducing magnesium and 0.5 mL of concentrated hydrochloric acid. The presence of flavonoids was indicated by the appearance of an orange color. Dragendorff’s and Mayer’s tests were performed for alkaloid detection. A mixture was created by combining 1 mL of MeOH extract with 0.5 mL of 2% hydrochloric acid. The resultant acidic solution was subsequently divided into two tubes. In Tube I, three drops of Dragendorff solution were added, while in Tube II, three drops of Mayer solution were introduced. Verification of the existence of alkaloids was established through the observation of a red or orange residue in Tube I and a whitish precipitate in Tube II. In terpenoid testing, MeOH extract (1 mL) was mixed with 0.5 mL of chloroform and then added with 0.5 mL of anhydrous acetic acid. After that, the mixture was carefully fed into a test tube with 1–2 mL of concentrated H2SO4, which presents a brownish or purple ring if triterpenoids are present.
After that, the mixture was carefully poured into a test tube, and 1–2 mL of concentrated H2SO4 was added. The Liebermann-Burchard reagent was used to test the presence of steroids by mixing MeOH (1 mL) with n-hexane (2 mL) and allowed to stand until two layers formed. The n-hexane layer was removed, and five drops of Liebermann-Burchard reagent were added. The presence of blue color indicated the presence of steroids. Saponins were identified through the combination of 2 mL of MeOH extract with 2 mL of distilled water, followed by allowing the mixture to stand for one minute. The addition of two drops of 1 N hydrochloric acid was performed, and the presence of saponins in the MeOH leaf extract was confirmed if foam developed and remained stable for 10 minutes.
The diet preparation and proximate composition process were explained elsewhere in Table 1. CO leaf powder was added to diets in the optimal dose (1.5 g/kg diet). This optimal dose was chosen based on the earlier study on tiger shrimp with a dietary proximate composition consisting of crude protein (38.21%), crude lipids (9.7%), crude fiber (4.52%), crude ash (12.48%), nitrogen-free extracts (32.86%), and total energy (16.46 kcal/kg).
This experiment was done at the farmer’s pond in Bonto Langkasa Village, Pangkep Regency, South Sulawesi, Indonesia. Post larvae (PL) shrimp in size 0.02 ± 0.02 g were purchased from a local hatchery. Then, shrimp was distributed to experimental ponds (500 m2) (170 shrimp/m2, duplicate) (Purnamasari et al., 2017). The stocking of PL was carried out in the morning, which begins with the acclimatization of PL first, especially at temperature and salinity. Once the temperature and salinity of the water within the PL plastic bag were either equivalent or exhibited minimal variation compared to the pond water, the gradual introduction of PL into the experimental ponds could be initiated. The shrimp were fed with a control and treatment diet for 45 days, followed by control feeding on both treatments, handed over to local farmers, and observation stopped. Before being used, the intensive ponds were prepared in accordance with the established procedures outlined in the REBAFE standard operating protocols. The trial feeding of 3% of body weight was carried out twice daily in the morning and evening for 45 days.
The shrimp were separately sampled to determine the shrimp growth performance during feeding. The collection of shrimp samples was conducted utilizing anchovy sampling technique. Anchovy sampling (n = 30) was conducted in the morning on the 45th day of the rearing period. Growth performance was evaluated by the following parameters: average body weight (ABW) (g) was measured by weighing the body weight of the sampling shrimp. Weight gain (g) = wt – w0. SGR (%/day) = 100 % × ([ln wt – ln w0] / t) where w0 is the initial mean body weight (g), wt is the sampling mean body weight (g), while t is rearing time (day).
Whiteleg shrimp that had been given a control diet (T1) and those fed a CO-supplemented diet (T2) for 45 days in the ponds were challenged with V. parahaemolyticus for 14 days. The shrimps from groups T1 and T2 were respectively selected 80 individuals each with an average weight of 7.0 ± 0.02 g and randomly assigned to control group (T1) and treatment group (T2) to evaluate the effects of CO on V. parahaemolyticus infection in shrimp. The experiment was conducted in eight rectangular plastic tanks (80 × 57 × 42 cm, capacity 150 L), each containing 20 shrimp. Each type of treatment was carried out four times (three for immune response and one for survival observation). The Fish Health and Management Lab of the Research Institute for Brackishwater Aquaculture and Fisheries Extension in Maros, Indonesia, supplied the pathogenic bacterium V. parahaemolyticus for the study. Determination of the density of V. parahaemolyticus reaching 104 CFUs/mL referred to Abdel-Tawwab et al. (2021) method. Whiteleg shrimps were infected with V. parahaemolyticus by injecting 0.1 mL intramuscularly into the ventral sinus. During the challenge test, the experimental diets were given twice daily in the morning and evening as much as 3% of body weight. To investigate how CO affect the growth of V. parahaemolyticus, the population of V. parahaemolyticus in the shrimp was determined using thiosulfate citrate bile sucrose agar media at 3, 6, 12, 24, and 48 hours after the challenge test.
Hemolymph samples were gathered both prior to the challenge test and at specific intervals post-challenge, specifically on the 2nd, 4th, 8th, and 14th days. The daily monitoring of the SR of whiteleg shrimp was conducted throughout the challenge test. The calculation of SR was performed using the formula as documented by Herawati et al. (2020). Prior to hemolymph collection, the shrimp were subjected to cold anesthesia. Hemolymph retrieval followed guidelines provided by Morales-Covarrubias et al. (2023) by using an anticoagulant solution instead of the modified alsever solution. The anticoagulant solution (100 µL) was inserted into a sterile syringe with a capacity of 1 mL. Then, with caution, the anticoagulant was mixed with hemolymph by taking 100 μL of hemolymph from the shrimp ventral sinuses on the base of the first abdominal segment. After hemolymph retrieval, the ventral sinus area of the shrimp was cleaned with a cotton swab that was soaked in 70% ethanol to prevent contamination. A mixture of hemolymph and anticoagulant (1:1) was placed in a 1.5 mL microcentrifuge tube by gently pipette up and down to prevent cell clumping. Next, trypan blue (0.5% in 2.6% NaCl) was used to dilute hemocytes. Hemocytes were examined under a light microscope (DP21, Olympus, Tokyo, Japan) at 10× magnification, employing a hemocytometer and introducing 10 μL of a diluted hemolymph sample. The THC (Fig. 1) was calculated according to the formula proposed by Braak (2002).
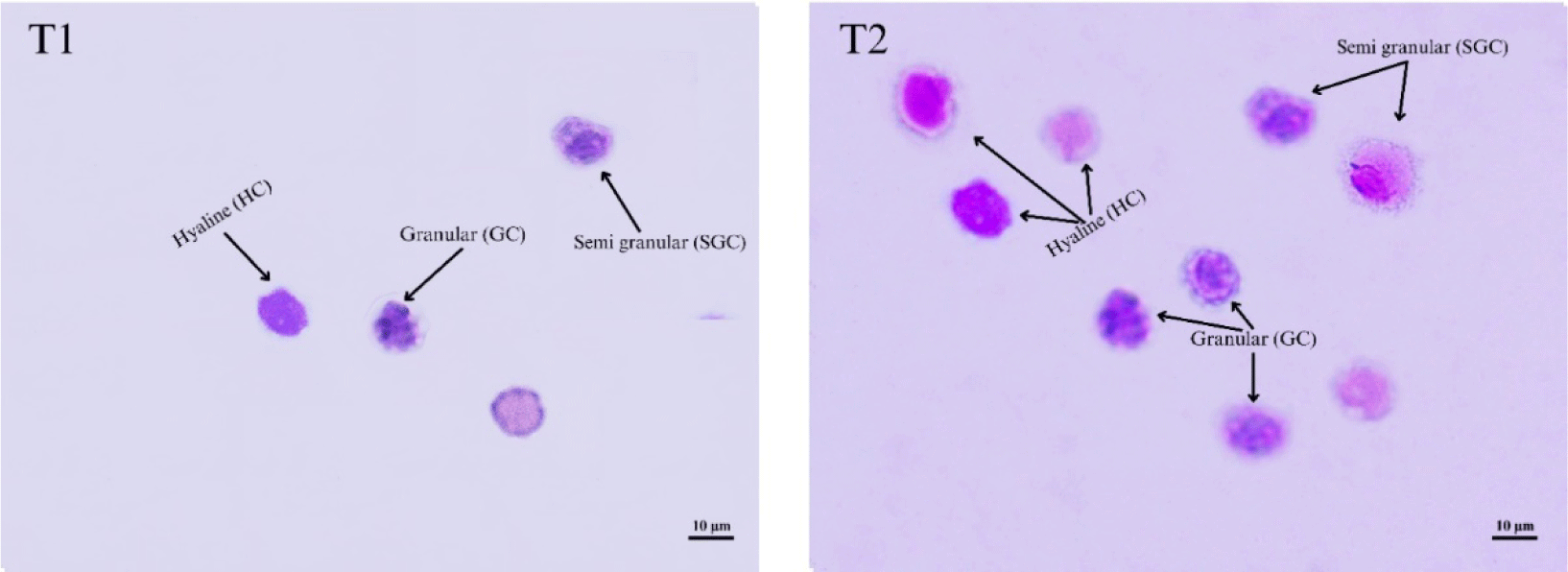
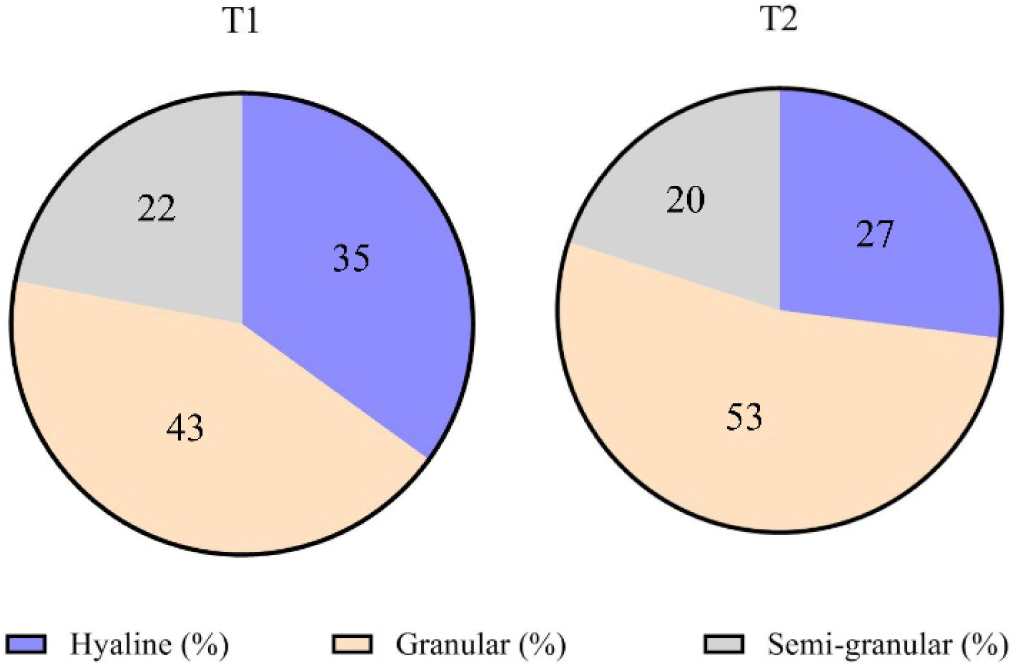
To calculate DHC, a microscope glass slide was prepared, and then 20 μL hemolymph from each shrimp (the remaining hemolymph was used to calculate THC) was placed on the slide. Hemocytes were allowed to flatten themselves and dry at room temperature, washed with pure MeOH for 15 minutes, and dried again at room temperature. Next, hemocytes were stained with Giemsa-Rosenfeld as much as 10%. Slide cleaning was carried out under running water for 30 seconds. Following the drying process, the slides were employed for the morphological characterization of distinct hemocyte types, as outlined by Chang & Chang (2022), utilizing a light microscope (DP21, Olympus) at a magnification of 40×. Diverse shrimp hemocytes, including hyaline, granular, and semi-granular, were recognized and quantified using the methodology described by Braak (2002). The DHC result (Fig. 2) was then calculated as the average of each hemocyte type in the total cells counted.
The activity of proPO was measured with the help of a spectrophotometer. L-dihydroxyphenylalanine was used as the base material to record dopachrome formation. To perform a proPO test, the following steps were performed. First, the prepared 100 μL of hemolymph was separated by centrifugation at 7000 rpm for 20 minutes at 40°C, and the supernatant was discarded. Then, a hemolymph precipitate was added to 100 μL of cacodylate citrate buffer solution (0.1 M C2H12AsNaO5, 0.45 M NaCl, and 0.01 M Na3C6H5O7) and then separated again in the same way. Next, the precipitate was resuspensioned in cacodylate buffer (100 μL) and homogenized. After that, 100 μL of the mixture was piped into a 96-well microplate (R-BioPharm AG, Darmstadt, Germany) and mixed with 100 μL of trypsin (Sigma-Aldrich, St. Louis, MO, USA). The mixture was then suspended and incubated at 25°C for 10 minutes. Enzymatic activity was measured by comparing the dopachrome’s absorbance and blanks containing trypsin (200 μL) and l-DOPA (50 μL, containing 1 mg/mL trypsin) at a wavelength of 490 nm.
During the experimental period, salinity, pH, Dissolved Oxygen (DO), and water temperature were measured every 10 days of the feeding trial at 08:00–10:00 Indonesian time using YSI 6920 V2-2 Multiparameter Water Quality Sonde (YSI, Yellow Springs, OH, USA).
Growth parameters, immune response (THC and proPO activity), and SR were evaluated through t-student test analysis using SPSS software version 20.0 (SPSS, IBM, Chicago, IL, USA) at a confidence level of 0.05 (p < 0.05), while DHC and water quality were presented in descriptive form. All Figures were rorganized using a Graphpad Prism versi 10 (GraphPad Softward, San Diego, CA, USA).
Results
The examination of phytochemical constituents confirmed the presence of flavonoids, alkaloids, triterpenoids, and steroids in the leaves of C. odorata. However, the MeOH extracts of C. odorata leaves did not exhibit the presence of saponins, as indicated in Table 2.
| Methanol extract | Flavonoid | Alkaloid | Triterpenoid | Steroid | Saponin | |
|---|---|---|---|---|---|---|
| Dragendorff | Meyer | |||||
| C. odorata L. | + | + | + | + | + | − |
This experiment showed that dietary CO supplementation had a positive impact in increasing the growth of whiteleg shrimp. Significant improvements (p < 0.05) in all growth parameters tested (Table 3) were shown by shrimp fed a CO-supplemented diet compared to shrimp fed a control diet after 45 days of feeding experiments with ABW of 7.71 ± 0.24 and 5.61 ± 0.22 g, respectively. A higher SGR of 13.23 ± 0.14% was noted in shrimp fed a CO-supplemented diet (T2), but a relatively low SGR (12.52 ± 0.17%) was recorded in shrimp fed a control diet (T1). The growth parameters measured were ABW, weight gain, and SGR. It suggests that CO can be used to improve the growth of whiteleg shrimp.
| Parameters | T1 | T2 |
|---|---|---|
| Average body weight (ABW) (g) | 5.61 ± 0.22 | 7.71 ± 0.24* |
| Weight gain (g) | 5.59 ± 7.78 | 7.69 ± 0.08* |
| Specific growth rate (SGR) (%/day) | 12.52 ± 0.17 | 13.23 ± 0.14* |
Based on the supplied data, the water quality obtained during the feeding trial was normal for whiteleg shrimp L. vannamei growth in the pond. The water quality measured consisting of salinity (ppt), pH, temperature (°C), and DO (ppm) was summarized in Table 4.
Adapted from Nguyen et al. (2002) with CC-BY-NC.
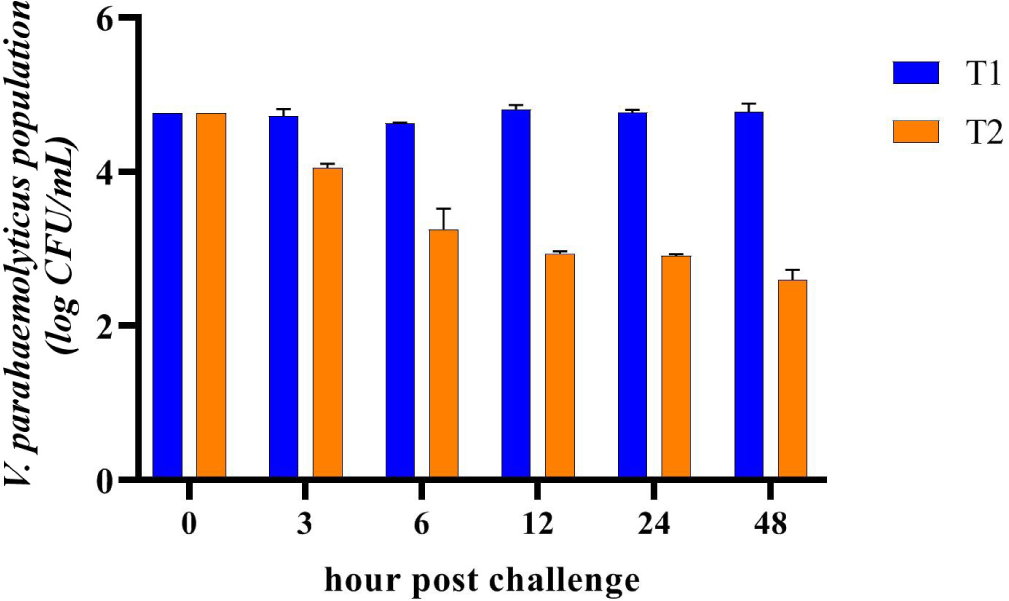
The treatment with CO (T2) had a significant impact on the growth of V. parahaemolyticus in the shrimp. As shown in Fig. 3, the population of V. parahaemolyticus (n = 3) in the shrimp fed a CO-supplemented diet exhibited a continuous decrease over time. After 48 hours of the challenge test, the population of V. parahaemolyticus decreased by 45.58%. In contrast, in the control group (T1), the population of V. parahaemolyticus in shrimp also experienced a decrease up to 6 hours after the challenge test, followed by an increase reaching its peak after 12 hours of the challenge test, and subsequently declined again but remained higher than the initial bacterial population.
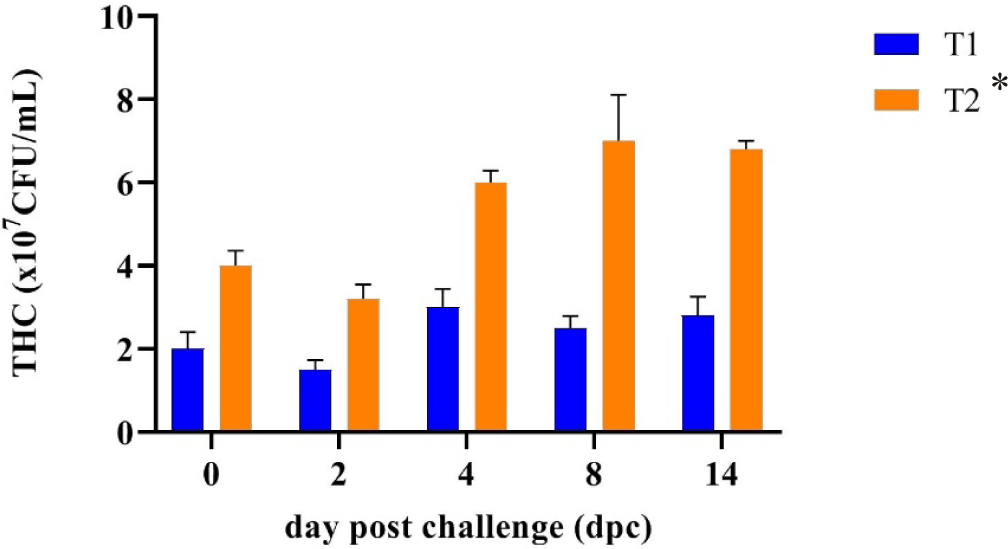
THC is a useful tool for assessing the health status of shrimp in aquaculture (Morales-Covarrubias et al., 2023). Fig. 4 shows that after a feeding trial for 45 days of farmed rearing, whiteleg shrimp fed a CO supplementation diet (T2) significantly increased their THC value (4 × 107 CFU/mL) compared to that (2 × 107 CFU/mL) of shrimp fed a control diet (T1). The result of the THC observation indicated that a higher THC in shrimp fed the CO-supplemented diet (T2) had been shown before the challenge test compared with the control shrimp (T1). THC levels were greatly reduced in shrimp fed the control diet may be because there had been an infection caused by pathogenic bacteria or viruses since being farmed.
After whiteleg shrimp were fed a CO-supplemented diet (T2) and control diet (T1) and challenged with V. parahaemolyticus, the proportions of the three hemocyte types changed (Table 5 and Fig. 5), and the percentage of GC increased dramatically. The pattern of changes in GC values in whiteleg shrimp in both treatments had the same tendency; however, GC values in shrimp fed diet T1 were always lower than GC values in shrimp fed diet T2. On the 2nd dpc, the granular cell number decreased in the T2 treatment and increased in the T1 treatment. Furthermore, on the 4th, 8th, and 14th dpc, the granular cell number decreased to the GC values lower than the baseline values in both shrimp treatments. The hyaline in the T1 treatment decreased on the 2nd dpc, then increased on the 4th, 8th, and 14th dpc. Unlike hyaline in the shrimp group (T1), the shrimp group (T2) increased on the 2nd dpc, then decreased on the 4th, 8th, and 14th dpc. Since the 2nd dpc, semi-granular in the T1 treatment decreased; conversely, semi-granular in the T2 treatment increased. The proportion change of the three hemocyte types was summarized in Table 5.
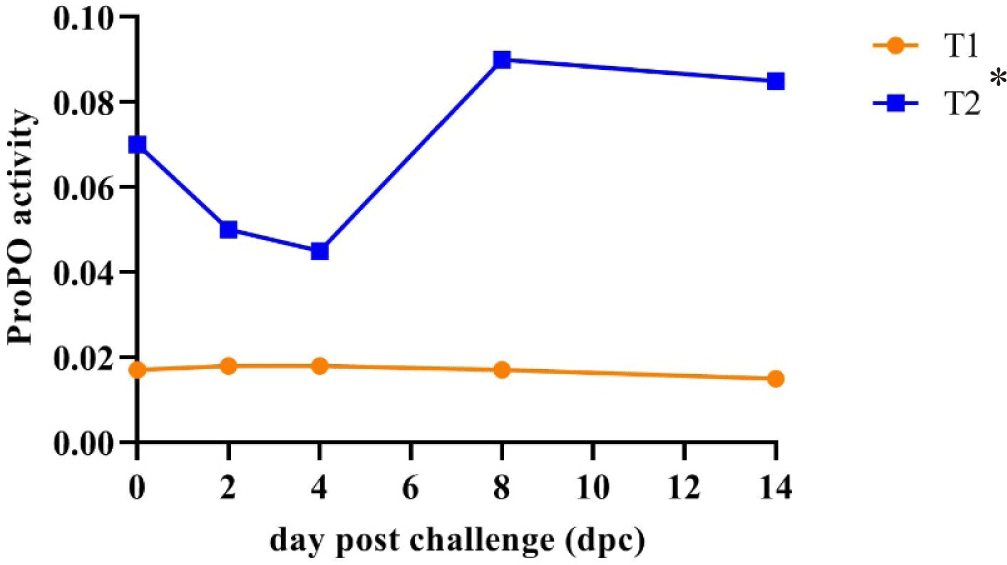
The current study found that the average proPO activity in shrimp fed the CO-supplemented diet (0.068) (T2) was higher and significantly different (p < 0.05) than that in shrimp fed the control diet (0.017) (T1). The proPO activity in the treatment shrimp (T2) decreased on the 2nd dpc, but their proPO activity was still higher than that in the control shrimp (T1) and then dramatically increased on the 8th dpc and remained relatively stable until the 14th dpc. In contrast, the proPO activity in control shrimp remained stable and lower than that of treatment shrimp during the challenge test (Fig. 6).
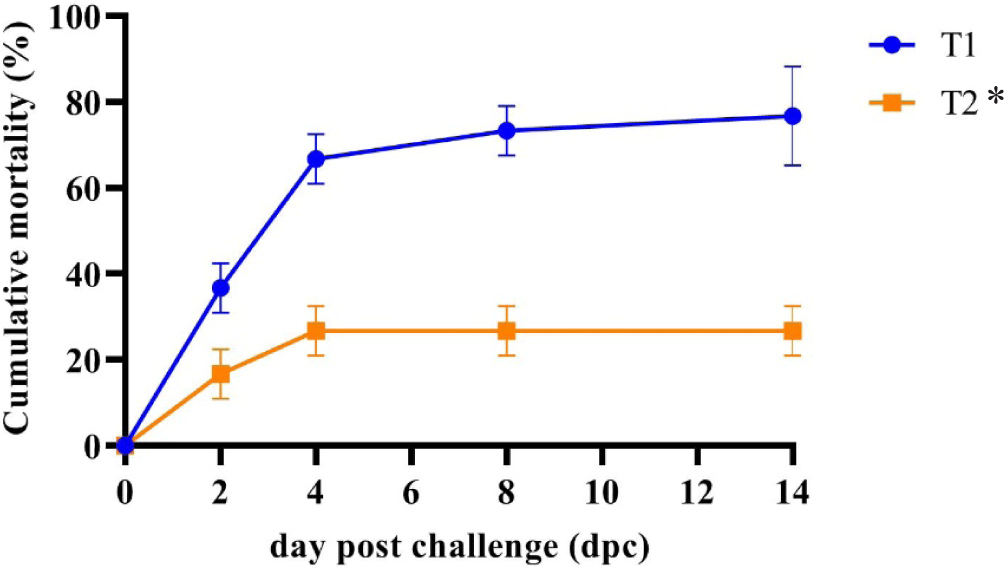
The mortality rate of shrimp receiving the control diet (T1) exhibited a sharp increase on the 2nd day post-challenge (dpc) and persisted until the end of the study, as illustrated in Fig. 7. Conversely, a consistently high survival trend was observed in shrimp fed the CO-supplemented diet (T2) from the 2nd dpc onward. By the 14th dpc, nearly all shrimp fed the control diet (T1) had succumbed. Between the 4th and 14th dpc, shrimp provided with the CO-supplemented diet (T2) demonstrated significantly greater resistance (p < 0.05) to V. parahaemolyticus infection (73.33 ± 5.77% SR) compared to those fed the control diet (T1) (23.33 ± 5.77% SR). This data indicated the increased survival of shrimp fed the CO-supplemented diet by 50%, which may be the effect of C. odorata leaf bioactive compounds.
Discussion
The study found that the MeOH extract of C. odorata leaves contains natural ingredients such as flavonoids, alkaloids, triterpenoids, and steroids. The specific chemical composition of C. odorata may vary depending on the species, season, and plant location. The presence of phytochemical compounds such as alkaloids, flavonoids, terpenoids, and steroids in the leaves of C. odorata was suspected to increase the immune parameters of crustaceans such as shrimp.
In this study, C. odorata leaf meal showed its efficacy in reducing V. parahaemolyticus population at 3 hours to 48 hours after the challenge test. The population of V. parahaemolyticus in the shrimp decreased by 45.58% or 3.90 × 102 CFU/mL after 48 hours of challenge testing (Fig. 3). Flavonoids modulate the immune system to enhance its function, whereas alkaloids serve as both immunostimulants and antibacterial agents, as documented by Idris Affandi et al. (2019). A similar result was also obtained by Behl et al. (2021) that the content of phytochemicals consisting of alkaloids, tannins and phenols has activity as an immunostimulant that can improve the quality of host immune cells. Flavonoids, alkaloids, and saponins have been shown to have pharmacological activity that can help protect whiteleg shrimp from bacterial diseases caused by Vibrio sp., called vibriosis (Nur et al., 2020; Santiago et al., 2021). Alkaloids, for example, boost shrimp’s immune system by enhancing the activity of macrophage cells, which can engulf and destroy pathogens.
After 45 days of rearing shrimp was farmed, the whiteleg shrimp fed with a CO-supplemented diet (T2) had a higher ABW, body weight gain, and SGR than those in the shrimp fed a control diet (T1). The shrimp increased its growth parameter because the CO-supplemented diet contained nutrients such as carbohydrates, protein, lipids, minerals, and bioactive compounds (Table 3) that promote growth (Aziz et al., 2020). C. odorata also contains bioactive compounds that function as antiseptic substances that can inhibit bacterial growth (Rasyid et al., 2020). The presence of flavonoids and alkaloids in the leaves of C. odorata, which have antioxidant and antibacterial properties, could help protect aquatic animals from oxidative stress and bacterial infection caused by environmental factors (Eze & Jayeoye, 2021). C. odorata leaf flour can be used as a food supplement for Bali cattle, as it contains nutrients that improve their consumption, digestibility, pH, N-ammonia, volatile fatty acids (VFA), and growth. Grape extract has been found to improve white shrimp’s antioxidant activity and growth performance, specifically L. vannamei (Chien et al., 2023a). The shrimp fed the CO-supplemented diet showed a weight after 45 days of rearing in the pond of 7.71 ± 0.24 grams (ABW). This ABW was higher than that (around 5.00 g) reported by Purnamasari et al. (2017). The daily growth rate (SGR) in this study reached 13.23 ± 0.14%/day, which was higher than the growth of whiteleg shrimp after being fed dry grape extract of 2.76 ± 0.06%/day (Chien et al., 2023b) and Seaweed Polysaccharide (7.59 ± 0.33) (Abbas et al., 2023). Several plant extract applications, such as Boesenbergia pandurata, Solanum ferox extract (Hardi et al., 2022), and Phyllanthus amarus extract, have been reported to enhance the growth of shrimp (Ngo et al., 2020). The increased growth rate of whiteleg shrimp obtained after 45 days of rearing could also come from pond water quality under normal conditions, as noted in Table 4.
Bioactive compounds in C. odorata increased immune responses (Harlina et al., 2019). The increased formation of phagocytic cells is essential in controlling the attack of microorganisms. Elevated THC levels are associated with increased cellular and humoral immune responses (Braak, 2002; Mulyadi & Iba, 2020). In this study, THC shrimp fed the CO-supplemented diet before facing the challenge test showed an increase in hemocyte counts compared to shrimp that were only given a control diet. It indicates a proliferation of hemocytes, hinting that the shrimp’s immune system has improved, making it better prepared to deal with pathogenic infections.
The average value of the THC post-challenge test of whiteleg shrimp fed the CO-supplemented diet (T2) (5.40 × 107 cells/mL) was significantly higher (p < 0.05) than whiteleg shrimp fed the control diet (T1) (2.36 × 107 cells/mL). This study showed that CO may have a better effect on whiteleg shrimp blood parameters after being challenged with V. parahaemolyticus than without CO. Decreased and increased THC levels can occur rapidly after a bacterial infection due to the body’s defence response. THC levels decreased on the 2nd day after a bacterial infection was detected and then increased again on the 4th day as part of the shrimp’s natural immune system recovery process as described in the study of Risjani et al. (2021). If the number of shrimps hemocytes increases, the body’s defences also increase to avoid infection. Conversely, hemocyte numbers decrease because many die after destroying foreign macromolecules when the infection is overcome and resistance to foreign bodies is achieved.
The high amount of shrimp THC after being challenged with V. parahaemolyticus showed that the C. odorata bioactive compound could increase the immune response by stimulating the production of immune cells and molecules, such as cytokines and antibodies (Vijayaram et al., 2022) and growth promotion. Shrimp are not equipped with adaptive immune systems, so they must rely on the innate immune system (Kumar et al., 2022). The high THC value found in the shrimp group (T2) could improve the disease resistance of shrimp to reduce the risk of infection, which has an impact on a higher survival (Fig. 7) in shrimp group T2 after being challenged with V. parahaemolyticus compared to the shrimp group T1. In the earlier study of different species, tiger shrimp given a diet supplemented with C. odorata leaf flour had a higher THC value than the shrimp group fed without C. odorata leaf flour (Harlina et al., 2019). Like the whiteleg shrimp in the current study, the THC value obtained was much higher, possibly due to the shrimp being fed a diet supplemented with CO for a longer time in the pond or different shrimp responses due to pathogens entering. THC values in shrimp before the challenge test with V. parahaemolyticus had shown differences. In a more general sphere, it is thought that environmental conditions play an important role in influencing crustacean immune responses and resilience, especially in shrimp farms where environmental factors such as laboratory environments are often difficult to control. Many reports have noted the use of plants to enhance non-specific immune parameters in crustacea (Hardi et al., 2022; Ngo et al., 2020).
DHC refers to the proportion of different types of hemocytes (HC, GC, and SGC) in the hemolymph of shrimp. These three hemocyte cell types played different roles in the immune system. Hyaline cells are responsible for executing the phagocytic role within the shrimp immune system, while the collaborative efforts of granular and semi-granular cells contribute to the synthesis and subsequent release of antimicrobial peptides. According to Xian et al. (2017), the presence of an immune response is indicated by changes in hemocytes. In this study, before the challenge test, shrimp fed the CO-supplemented diet (T2) during the 45-day rearing period showed lower values of semi-granular cells and hyaline cells than these values in shrimp fed without CO-supplemented diet (T1) (Table 5 and Fig. 5). The lower semi-granular cells obtained could indicate the possibility that the bioactive compounds of C. odorata leaf triggered the maturation of hemocyte cells more quickly than in control shrimp during the grow-out in the pond. The change in the number of hyaline cells is closely related to the semi-granular cell number derived from late-stage hyaline cell development. Thus, a reduction in the quantity of semi-granular cells is observed, attributed to the incapacity of hyaline cells to undergo differentiation into semi-granular cells (Risjani et al., 2021).
The number of differential hemocyte cells during the challenge test changed over time, and it can be found that hyaline and granular cells may play a key role in immune system function. The smallest cell type is hyaline cells, with a high ratio between nucleus and cytoplasm and relatively few cytoplasmic granules. These cells play a crucial role in the body’s immune defense system, with the quantity of hyaline cells typically rising in correlation with heightened phagocytic activity. In instances of hyaline cell infection, a notable increase is commonly observed as an initial response in the body’s defense mechanisms (Risjani et al., 2021). In this study, shrimp hyaline cells in T1 treatment groups decreased on the 2nd dpc, then increased on the 4th dpc, and continued to increase until the 14th, indicating an infection in hyaline cells.
In contrast, hyaline cells in the T2 groups increased on the 2nd dpc and then decreased on the 4th, 8th, and 14th dpc; instead, semi-granular cells continued increasing, indicating shrimp recovery from the infection. The granular and semi-granular cells are involved in crustaceans’ recognition and defence system. Bioactive compounds of C. odorata leaf flour had increased shrimp protection from V. parahaemolyticus infection by increasing semi-granular cells associated with continuously increasing semi-granular cells since the 2nd dpc. Similar results have been reported in the use of Xylocarpus granatum leaf extract, which increased the number of semi-granular cells in tiger shrimp tested with V. harveyi by as much as 1.55 times higher than semi-granular cells in control shrimp (Saptiani et al., 2020).
The proportion of granular cells predominates among the three hemocyte cells (Fig. 2). The shrimp fed a CO-supplemented diet had granular cells of 53%, higher than the granular cells in the shrimp fed without a CO-supplemented diet (43%). The highest percentage of granular cells, also known as neutrophils, serve as pioneers in immune defence against Gram-negative bacteria; granular cells in this study indicated that bioactive compounds of C. odorata leaf could increase the srimps’immune system by inducing hemocyte improvement (Xian et al., 2017). They have several mechanisms to enhance the immune response, including phagocytosis, degranulation, and NETosis. Neutrophils can engulf and kill pathogenic bacteria through multiple bactericidal pathways. In addition, they have granules and enzyme pathways that help to eliminate pathogenic microbes. These granules can release cytokines that activate antigen-presenting cells and amplify the immune response.
Furthermore, neutrophils can produce lysozyme, an antimicrobial enzyme that can kill certain bacteria independently of peptidoglycan hydrolytic activity and modulate the host’s immune response to infection. Dietary supplementation with methionine (Met) can improve growth performance and increase the activities of antioxidant enzymes in shrimp (Huang et al., 2020). Hyaline and granular cells were the two dominant cell types that were always observed to be higher than semi-granular cells after the 4th, 8th, and 14th dpc. The same pattern on both cell types was observed using seaweed extract Sargassum sp. to improve immune parameters (THC and DHC) in white shrimp L. vannamei juveniles challenged with V. algynolyticus (Mulyadi & Iba, 2020).
The proPO system’s activity is a key element in the immune response associated with the defence against pathogens and their removal (González-Rete et al., 2019). The proPO cascade is triggered by a small number of microbe-derived molecules such as lipopolysaccharides, β-1, 3-glucans, or peptidoglycan, which are recognized by pattern-recognition proteins. ProPO is identified as a critical element within the immune system of shrimp, serving a pivotal role in safeguarding against microbial infections. PO, a crucial enzyme of the proPO system in invertebrates, promotes humoral defence mechanisms and subsequently eliminates pathogens. The proPO activity in the shrimp fed a CO-supplemented diet during the 45-day culture period in the pond was much higher than in the shrimp fed the control diet. This result is like previous studies that obtained higher proPO activity in shrimp who received diet supplementation with herbs material/extract (Ngo et al., 2020). The proPO activity enhancement in treated shrimp hemolymph (T2) indicates an increased immune response to the pathogen, which may impact shrimp SR of 73.33%.
On the other hand, the low SR observed in the control group (23.33%) (T1) may be due to the low activity of proPO. The decreased proPO activity can cause failed phagocytosis and tissue damage. A similar finding of the proPO activity in whiteleg shrimp was obtained in tiger shrimp when challenged with V. harveyi. The C. odorata leaf flour-supplemented diet significantly increased the proPO activity in the treatment tiger shrimp group compared with the control tiger shrimp group (without C. odorata leaf flour supplementation) (Harlina et al., 2019).
Conclusion
The leaves of C. odorata were confirmed to contain bioactive components such as alkaloids, flavonoids, triterpenoids, and steroids, which are suspected to improve shrimp health. L. vannamei that received the CO at 1.5 g/kg of diet showed improved growth parameters and enhanced resistance to V. parahaemolyticus infection. The heightened THC, along with increased levels of granular and semi-granular cells and proPOpost the feeding trial, are associated with the supplementation of CO in the diet. The findings of this investigation propose that these dietary components have the capacity to serve as immunostimulants in shrimp, consequently enhancing growth and bolstering the immune response, thereby augmenting the resistance of whiteleg shrimp against bacterial infection. These results provide useful information for sustainable shrimp culture and the potential use of CO as a shrimp diet additive to improve growth, survival, immune responses, and resistance to bacterial infection. To explore the future perspectives of how the specific phytochemicals in CO stimulate the immune system, further research and investigations involving the study of the mechanisms of action of these phytochemicals, their interactions with the immune system components, and their effects on immune-related gene expression or signaling pathways in shrimp are needed.