Introduction
Freshwater rivers have very important ecosystem services, provisional, regulations, supporting, cultural and religious activities (MEA, 2005). Fish is one of the provisional ecosystem services that has both ecological and commercial importance in aquatic ecosystem and for human needs (MEA, 2005).
Ethiopia has many lakes and rivers which are small to large size. Though the actual size of these water bodies were not documented, roughly, the country has about 7,400 km2 of lake area and about 7,000 km of river length (Wood & Talling, 1988). Recently new dams like Grand Ethiopian Renaissance Dam and other small and medium sized dams are being constructed for hydroelectric and irrigation activities. In East Africa, Ethiopia is the water-tower and endowed with several water bodies that contain a high diversity of fish (Golubtsov & Darkov, 2008; Golubtsov & Mina, 2003).
Ethiopia has no long history in fish species investigation. The first review, conducted by Shibru (1973) reported 93 fish species. Following this, other researchers, Getahun and Stiassny (1998) reported 65 fish species from Blue Nile Basin, Getahun (2005); Stiassny & Getahun (2007) reported about 193 fish species (153 indigenous, 40 endemic and 10 exotic species) in different freshwater bodies of the country and Golubstov & Darkov (2008) reported 113 fish species from six major drainage basins of Ethiopia including Blue Nile. Since 2006, fish species investigation in rivers of Ethiopia has been conducted by different MSc students mainly by Addis Ababa, Bahir Dar and Wollo Universities. Bahir Dar Fishery and Other Aquatic Life Research Centers has also played significant role in fish species investigation. Although, the fish species investigation is not complete, the diversity of fish species in Ethiopia is estimated to be more than 200 species (Mengesha, 2015; Tesfaye & Wolff, 2014).
Despite the above efforts made on fish species investigation, many of small, medium and large rivers of the country are not fully explored (Getahun, 2017; Mekonen & Hailu, 2021; Mengesha, 2015). Terie River is one of such rivers that have been poorly explored. There was no exhaustive systematic research has been done on the diversity, abundance and biology of fishes in Terie River, one of the major rivers in Blue Nile basin originated from the highlands of Wollo area. Therefore, this study aimed to assess the fish diversity, determine length weight relationship and Fulton condition factor (FCF) of fishes and assess some physicochemical water quality parameters of the river. The findings are expected to enhance knowledge on fish diversity and biology of fishes in unexplored rivers like Terie River. The findings will be also used as baseline data for further fish biodiversity investigation.
Materials and Methods
Terrie River is one of the major tributaries of Beshilo River. Beshilo River is one of the 16 major tributaries of Abay (Blue Nile River). Teri River joins Beshilo that joins Abay River (Fig. 1). Terie River is located in Delanta District of South Wollo Zone of Amhara Region, Northeastern Ethiopia.
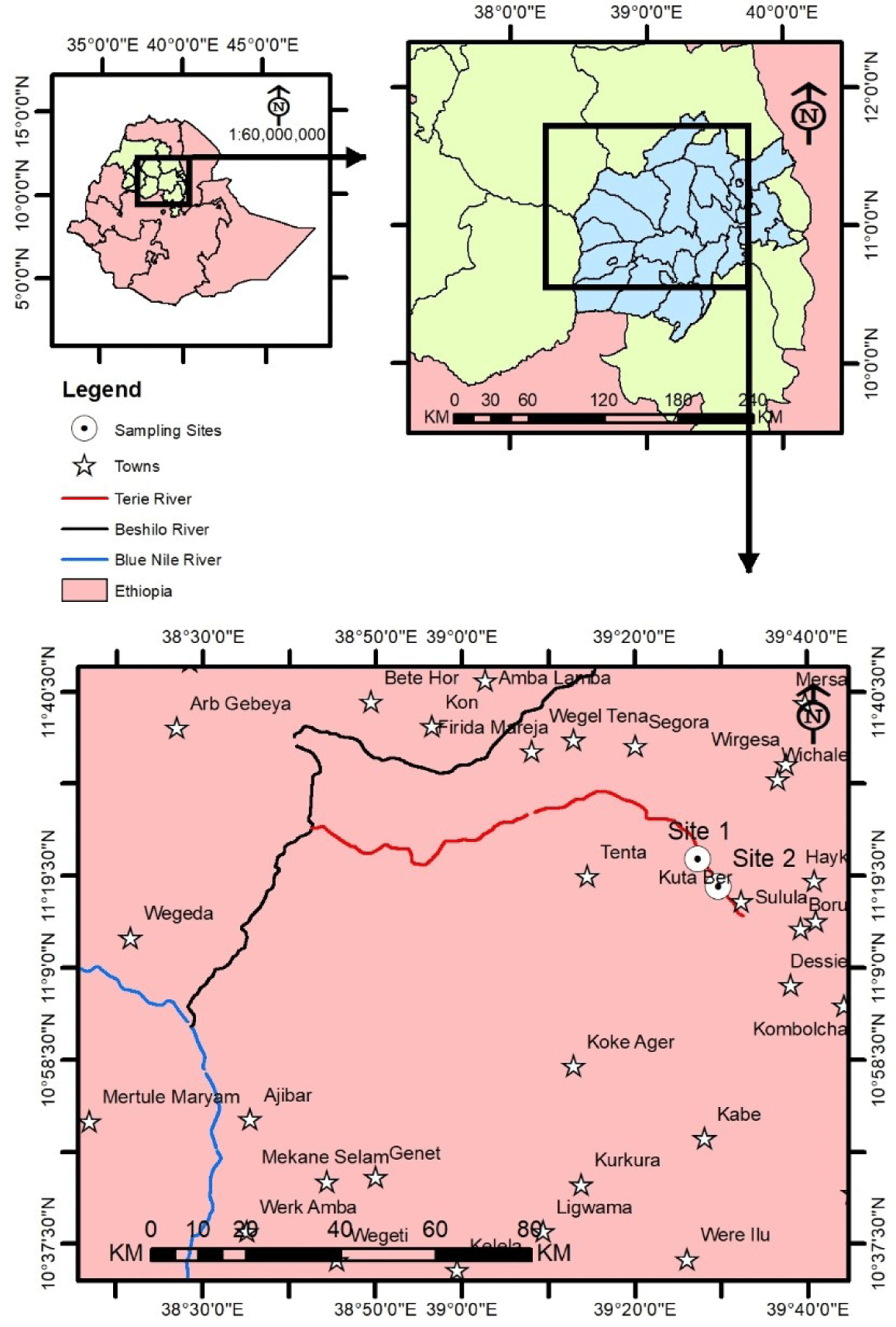
“Terie” River is formed from Dry River (“Derek wenz”) and “Masoria” River. Both of them originate at the higher areas of Woldya. “Derek wenz” begins around Mersa and it is called ‘Workiju’ (the Gold hand). “Masoria” River is relatively big river that originates from the high lands of “Woldya” called “Anbesa Maseria”. When “Masoria” River goes down receiving water along its way, it gets larger and wider and now known as ‘Tirgia’ (meaning large and strong river). Tirgia and “Workiju” Rivers join and form “Terie” River at a distance of about 2 km from the origin of Beshilo River (Mogous, 2007).
In this study, two sampling sites were selected based on the habitat type (depth and nature of bottom substrate type), accessibility and fish population. Site 1 was shallow in depth and muddy bottom substrate whereas Site 2 was deeper and rocky bottom substrate. The geographic locations of Site 1 and Site 2 in UTM were (X: 549844, Y: 1255489) and (X: 554133, Y: 1249735), respectively. Each sampling site was sampled twice during the dry season (January) and twice during the wet season (April) in 2022. Gillnet of stretched mesh size of 4, 6, 8 and 10 cm were used to sample fish by setting the gillnets. Total length and weight of all specimens of fish were measured. Pictures were taken for each species. After taking all the necessary information individual specimen was preserved with 4% formalin in plastic jar and transported to the Wollo University laboratory for further identification and measurement.
In Biology department, Wollo University, specimens of fish were soaked in tap water for one day to wash the formalin and were identified by identification keys (Getahun, 2017; Golubtsov et al., 1995).
The diversity of fish was determined using the Shannon diversity index (H′). The Shannon index of diversity is a measure of species weighed by the relative abundance (Begon et al., 1990). Shannon index of diversity (H′) was calculated using the formula below:
where pi is the proportion of individuals in the ith species. Shannon index was used to indicate diversity of fishes at different sampling sites or rivers.
The relationship between total length and total weight of most dominant fish (O. niloticus and L. intermedius) was calculated using power function as in Bagenal & Tesch (1978).
where TW is the total weight (g), TL is the total length (cm), a is the intercept of regression line, b is the slope of regression line.
The wellbeing of each dominant species was studied by using FCF (Bagnal & Tesch, 1978).
where TW is the total weight in gram and TL is the total length in cm.
A total of nine physicochemical water quality parameters (pH, dissolved oxygen (DO), water temperature (WT). Conductivity, TDS, nitrate, phosphate, ammonia and Secchi disk) analysis were done in both dry and wet seasons from the two sampling sites. The pH, DO (mg/L), WT (°C) and conductivity (µS/cm) were measured using Wagtech portable multimeter, nitrate (mg/L), ammonia (mg/L) and phosphate (mg/L) were analyzed with Palintest photometer 7,500 model and the Secchi Disk depth (cm) was measured using Secchi disk apparatus.
Fish diversity and length-weight ratios were determined using Shannon index and regression, respectively. In addition to this, tables and frequencies were used to summarize the data. Independent t-test was used to compare the mean physicochemical water quality parameters difference between seasons and sites. SPSS 20 and Excel 2010 were used to conduct the descriptive and inferential statistics.
Results and Discussion
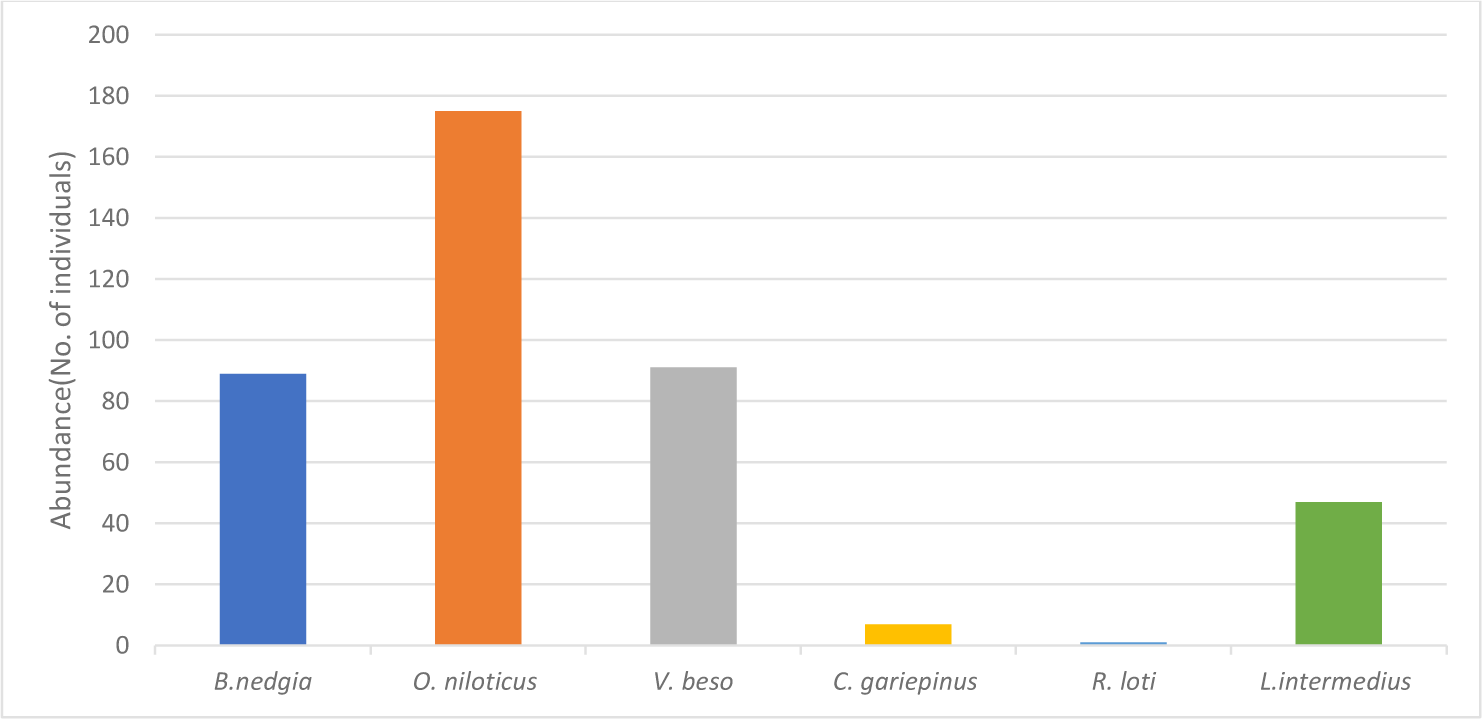
A total of six fish species, Labeobarbus nedgia, Labeobarbus beso, Ramius loti, Labeobarbus intermidus, O. niloticus and Clarias garipinus were recorded from Terie River. The first four fish species are members of the family Cyprinidae, the other fish species are member of Cichlidae and Clariidae, respectively (Table 1). In the present study, number of fish species were more in dry season (H’ = 1.36) than wet season (H’ = 0.49). The fish species diversity also varied with sampling sites, more fish species in deeper and rocky site (six species) and only one species in muddy and shallow site. Therefore, the fish species diversity was different between sites and seasons. The higher fish species in dry season could be due to less challenge from water velocity (less), availability of pools for feeding and reproduction and better water quality for their biology though the clarity of the water in dry season also has some challenges such as exposure to predation and fishing mortality (Gebremendhin & Mingist, 2014; Getahun et al., 2020; Tewabe, 2008). The lower fish diversity in wet season could be due to high rainfall, wood logs, leaves and roots which were brought by flooding could have decreased the efficiency of gill-nets and some less tolerant fish species could be washed away by the flood. This result is in consistent with previous study conducted in some rivers of Ethiopia, Upper Millie (Tessema et al., 2012; Yizezew, 2023), Lower Millie (Hussein, 2023) and Aveya River (Gebremendhin & Mingist, 2014).
The fish species diversity of Terie River was lower than those reported from some other freshwater rivers of Ethiopia. For instance, 57 fish species from Baro and other tributary rivers in Gambella Region (Tesfaye & Wolff, 2014), 27 species from Guang, Shinfa and Aymen Rivers in Blue Nile Basin (Tewabe, 2008), 23 fish species from the Beles and Gilgel Beles Rivers (Getahun et al., 2020), 13 species from Gilgel Abay and Andassa Rivers (Aynalem et al., 2018) and 17 species in the head of the Blue Nile River (Oumer et al., 2011). However, the fish diversity of Terie River was higher than Upper Millie River (four species; Yizezew, 2023) but equivalent with Lower Millie River (six species; Hussein, 2023). The lower fish diversity in the present study might be due to difference in intensity of fishing and efficiency of fishing gears (Limbu et al., 2018; Parvez et al., 2017).
The diversity of fish species was more in deeper and rocky site than in shallow and muddy site. The higher fish species in deeper and rocky site could be due to more suitable refugee site, access to hide under rocks and going deep to escape from predation and fishing mortality than the shallow and muddy site. The fish species diversity was also varied between sampling sites, more in Site 1 than site 2. The difference in fish diversity between sites could be attributed to habitat selectivity, change in catchment area, canopy closure, substrate type, distance from source, depth and width of rivers (André & Teugels, 1998; Getahun et al., 2020).
The abundance of fish species varied between seasons; fish were more abundant in dry season than wet season. In dry season, O. niloticus was the most dominant (175) and R. loti was the least abundant (1) (Fig. 2). In wet season, L. intermidus was more dominant (206) than O. niloticus (50).
Fish abundance may vary seasonally due to different factors. For instance, variation in available nutrients and habitats, temperature, water level, turbidity, fishing effort, fish behavior, size and life history stages of fishes, sampling seasons and altitude could have impact on fish abundance in rivers (Graaf, 2003; Karenge & Kolding, 1995; Mengesha, 2015; Zinabu et al., 2002).
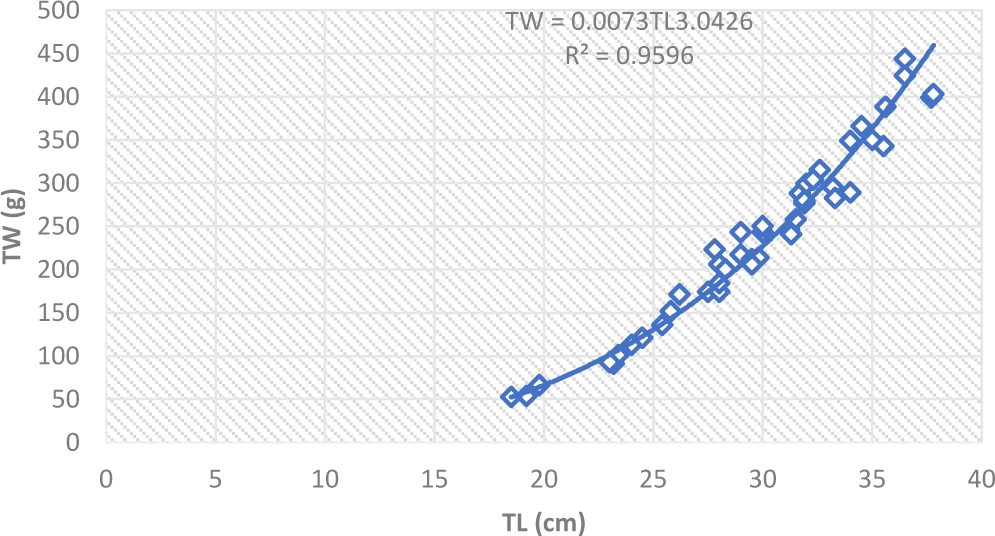
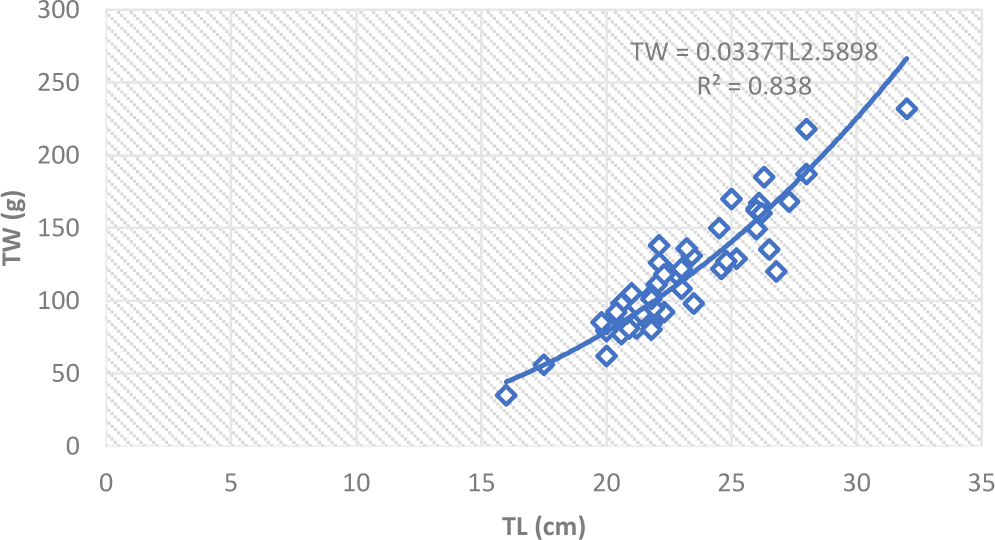
The regression coefficient value (b) in fishes may be nearly 3, indicating the fishes retain the same shape and their specific gravity remains unchanged during their life time (Joung et al., 2008). If the weight increased according to the fish length, it is said to be isometric growth (Raje, 2006). But, the b value in fishes may be greater or less than 3 showing allometric growth condition (De Graaf et al., 2005). The length and weight relationship of L. intermedius were TW = 0.0073 TL3.04 in dry and TW = 0.0337TL2.59 in we season indicating isometric and negative allometric growth, respectively (Fig. 3). While O. niloticus showed negative allometric growth, TW = 0.096TL2.44 and TW = 0.0335 TL 2.76 in dry and wet seasons, respectively (Fig. 4). In the present study, the b value of L. intermedius was greater than the same species (2.5) in Geba and Sor Rivers in White Nile Basin (Simagegnew et al., 2017) and comparable (2.96) with the species in Angereb and Sanja Rivers, Tekeze Basin (Tesfaye, 2006). In the present study the b value of O. niloticus was lower compared to the value (3.1) reported for Koka Reservoir (Berhan et al., 2019). But it was higher than the value (0.47) reported from Wudil River in Nigeria (Getso et al., 2017).
The mean FCF of L. intermidus were 0.84 ± 0.03 and 0.94 ± 0.25 in dry and wet seasons, respectively (Table 2). In the present study, the FCF of L. intermidus was comparable (1) with the same species in Beles and Gilgelbeles Rivers, Nile basin (Tesfaye, 2006). But it was lower (1.21) in Geba and Sor Rivers, White Nile System (Simagegnew et al., 2017) and (1.23) in Borkena River, Awash Basin (Tessema et al., 2012).
| Season | Species | Mean | SE |
|---|---|---|---|
| Dry | Labeobarbus intermedius | 0.844 | .026 |
| Oreochromis niloticus | 1.708 | .013 | |
| Wet | Labeobarbus intermedius | 0.943 | .025 |
| Oreochromis niloticus | 1.730 | .012 |
The mean FCF of O. niloticus was 1.70 ± 0.13 and 1.73 ± 0.12 in dry and wet seasons, respectively (Table 2). The FCF of both O. niloticus and L. barbus were higher in wet season than dry season. The FCF of O. niloticusin Terie River was higher (1.66) reported from Koka Reservoir in Ethiopia (Berhan et al., 2019). The FCF value of the present study was comparable with the value (1.73) reported from Tekeze Reservoir in Ethiopia. However, it was lower than the value (1.80) reported for Wudil River in Nigeria (Getso et al., 2017).
The more FCF in both fish species in wet season could be attributed to the availability of more food and important nutrients washed and accumulated in the sampling site. The Chi-square analysis showed that there was no significant difference in FCF between season (Chi-square = 899, df = 420 and p > 0.05). The condition factor is a good indicator of fish wellbeing’s. It shows the well-being of fishes in their natural habitat or in aquaculture and it is represented by the coefficient of body condition. It is an indicator of different biological and ecological factors in relation to fish feeding habits (Nehemia et al., 2012). Better body condition is correlated with high values of condition factors. Similarly, poor body condition is obtained when the values of the condition factor are less (Gupta & Tripathi, 2017).
The independent t-test analysis of physicochemical water quality parameters showed that there was significant difference in DO, Secchi disk depth, water temperature, NH3 and conductivity between seasons (Table 2). However, only conductivity and TDS were statistically significant between sampling sites (Table 3).
Water quality parameters especially temperature, DO and pH are the key factors to regulate the fish biodiversity and populations in freshwater bodies (Sarkar & Saha, 2021). Water transparency and electrical conductivity have also very important role in species diversity. Different fish species prefer different habitats due to the difference in their ecological requirements (Huang et al., 2019). Knowledge on the spatial fish assemblages in relation to their water quality is critical for fish assemblage management and conservation (Zona et al., 2022). Hydromorphology and DO were more important for fish diversity. Substrate and riparian habitat quality (refuge habitat and as pollutant) could also determine the fish diversity and population (Comte et al., 2010; Posthuma & de Zwart, 2005; Yuan & Norton, 2003).
The mean concentration of DO in the present study was (5.13 mg/L) in dry and (3.90 mg/L) in wet season. The lower DO in wet season could be attributed to more nutrient and silt load. In wet season the DO was slightly lower than the recommended limit for fishes (> 4 mg/L) (Shrestha & Basnet, 2018; UNEP, 2018). Low levels of DO are often linked to fish kill incidents. On the other hand, optimum levels can result to good growth, thus result to high production yield (Table 4).
The mean pH of Terie River was 8.79 in both dry and wet season indicating it was in alkaline range and was in suitable water quality standard range (6.5–9.0) for aquatic life (UNEP, 2018; Woynarovich et al., 2020). The pH of an aquatic ecosystem is important because it is closely linked to biological productivity.
The concentration of NH3 in Terie River was around 2.2 mg/L which was higher than the recommended limit (< 0.015 mg/L) (UNEP, 2018). The higher NH3 in this study could be due to the more nutrient and sediment load generated from the nearby geology and agricultural activities. High concentrations of ammonia could be the cause of higher pH and ammonia concentration in the blood of the fish which can damage the gills, the red blood cells, affect osmoregulation, reduce the oxygen-carrying capacity of blood and increase the oxygen demand of tissues (Lawson, 1995). In the present study, except NH3 the mean physicochemical water quality parameters of Terie River were within the surface water quality permissible limit for aquatic life as shown in Table 5.
| Parameter | Present study | UNEP (2018) | Woynarovich et al. (2020) |
|---|---|---|---|
| DO (mg/L) | 4.5 | > 3 mg/L | > 4 |
| pH | 8.8 | 6.5–9.0 | 8.3–9.0 |
| Tem (°C) | 27.6 | ND | 23–33 |
| Trans (cm) | 9.4 | ND | 30–50 |
| Cond (µS/cm) | 323.4 | 1,000 | 1,000–2,700 |
| PO4 (mg/L) | 1.0 | ND | 0.6–1.8 |
| NO3 (mg/L) | 3.4 | ND | < 40 |
| NH3 (mg/L) | 2.2 | 0.015 | ND |
| TDS (mg/L) | 170.8 | ND | < 500 |
Conclusion
From Terie River, five commercially important fish species, O. niloticus, L.intermidius, C. gariepinus, L. nedia and L. beso and one ecological important fish species, R. loti, were identified. The number of fish species diversity and abundance could be increased if we could be able to increase the sampling sites and sampling period. Except ammonia, all the physicochemical water quality parameters of Terie River were within the aquatic life permissible standard. The most preferred commercially important fish species, O. niloticus in the region and in the country as a whole is very abundant in the river. However, the communities in the vicinity have no interest to use the fish for food. This study is the first report which can be used as baseline. However, intensive study, more sampling sites and extended sampling period should be conducted to get full picture about the fish species diversity and biology of fishes of Terie River. Awareness creation should be also conducted about the importance of fishes for fish nutrition and livelihoods.