Introduction
Goby is a highly diverse group of fish with a widespread distribution globally, exhibiting significant variations in morphology, ecology, and behavior (Keith et al., 2015). It represents most of the biodiversity of freshwater systems in the islands of the Indo-Pacific region (Keith et al., 2021). The most prominent fish families in these island river systems are the Gobiidae and Eleotridae (Keith et al., 2021), as they primarily consist of freshwater spawning amphidromous species (Mennesson et al., 2015). Goby amphidromous spawns in freshwater, and the free-floating embryos drift downstream to the sea, where it undergoes a planktonic phase. Subsequently, goby amphidromous returns to rivers for growth and reproduction (Lord et al., 2012).
Goby-fry fishery has been well-documented in various locations worldwide, including the Caribbean Sea, Pacific Ocean, and Indian Ocean (Castellanos-Galindo et al., 2011). This group of species has widely recognized ecological and social value, but many are at increased risk of local, regional, or global extinction (Arthington et al., 2016). The fishery is often overlooked and neglected, even at the national level, where it serves as an alternative to the consumption of animal protein at the local level. Additionally, goby-fry contributes important periodic inputs to local freshwater and marine food webs (Castellanos-Galindo et al., 2011). According to Nurjirana et al. (2022b), the lack of attention and proper management efforts in the fishery can have severe implications for the sustainability of goby species in the future. These conditions exacerbate the biological fact that goby fish has a complex life cycle (Keith et al., 2015; Mennesson et al., 2015).
Regional-level studies on goby fishery plays a crucial role in shaping future policies and approaches concerning the utilization, population quantities, and inter-island connectivity. These studies provided valuable insights that supported the implementation of regional management strategies aimed at conserving the important species (Frotté et al., 2019). Tomini Bay, situated along the Wallacea-Weber line between the Pacific and Indian Oceans, is the largest bay in Indonesia, boasting a remarkable diversity of aquatic resources and a rich potential for ichthyofauna. One potential fishery of Tomini Bay is goby-fry, which is widely known as duwo or Nike (local name). Olii et al. (2019) identified goby-fry species based on genetics in Gorontalo Bay. Additionally, Sahami et al. (2019) and Sahami et al. (2020) studied the genetic diversity of goby-fry in the area. Studies on goby-fry diversity in Paguyaman Bay, the estuary of the Paguyaman River, were carried out by Sahami & Habibie (2021).
Efforts to evaluate genetic variation and propose protective measures are crucial, specifically in the face of significant changes in the habitat environment that can threaten the survival of fish species (Shen et al., 2016). This study carried out genetic identification to overcome the ineffectiveness of traditional morphological identification in goby-fry without distinctive morphology (Shen et al., 2016). The result contributed to the knowledge of the distribution of Indonesia ichthyofauna. Salam et al. (2016) stated that at least 4 rivers flowed into the waters of Tomini Bay, namely Bone-Bolango, Taludaa, Paguyaman, and Marisa. The presence of these rivers indicates a wider distribution of gobies. Therefore, this study aims to identify the goby-fry and create the distribution map in the waters of Tomini Bay Gorontalo.
Materials and Methods
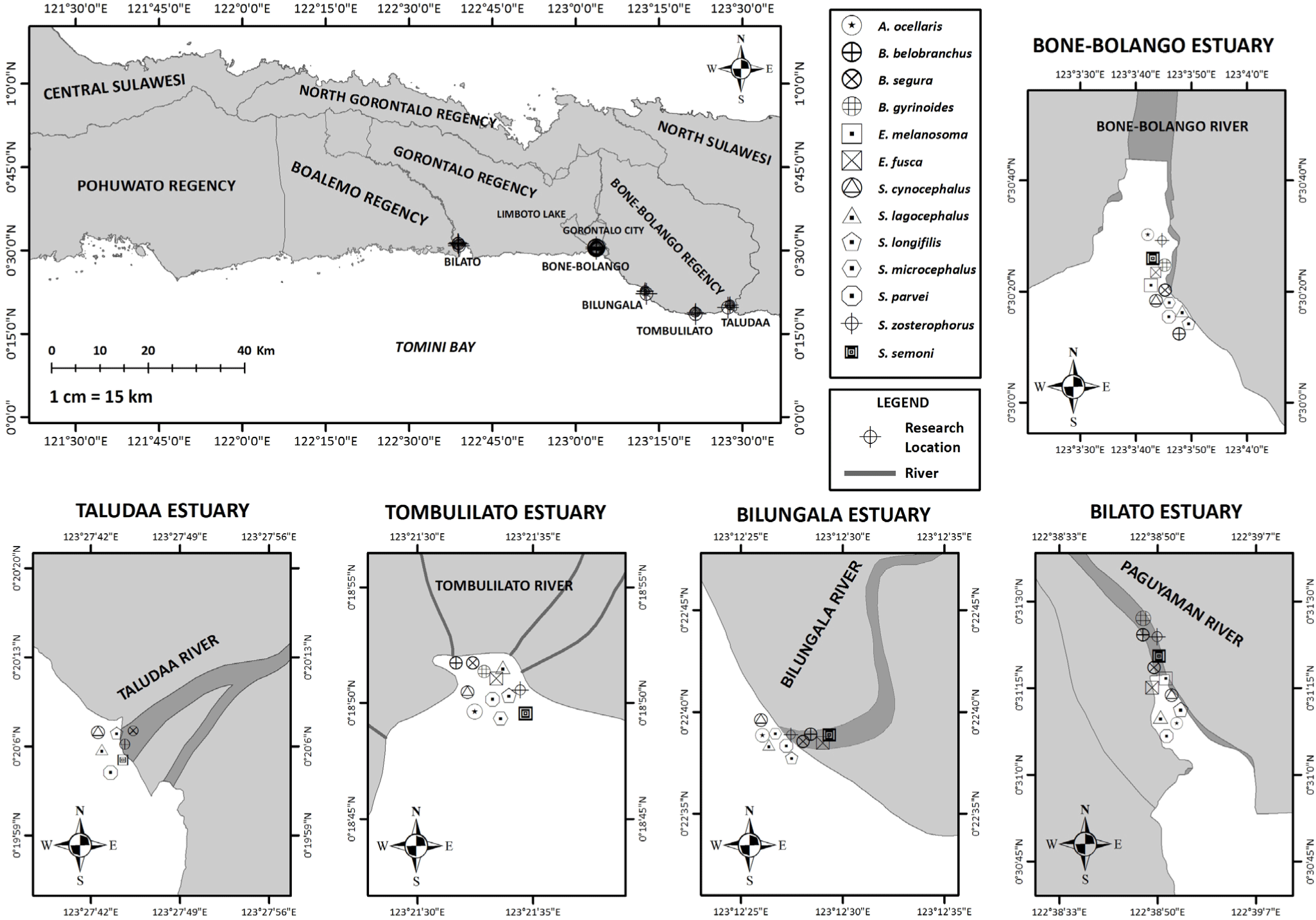
Sampling was conducted in 5 river estuaries in Tomini Bay, Gorontalo, namely Bone-Bolango (0°30’15.30”N–123°3’42.24”E), Paguyaman (0°30’41.48”N–122°39’8.00”E), Bilungala (0°22’ 37.24”N–123°12’27.35”E), Tombulilato (0°18’28.11”N–123°20’53.71”E), and Taludaa (0°20’6.57”N–123°27’42.05”E). A total of ± 150 mL goby-fry was taken from fishermen catches through the use of traditional fishing gears, locally known as dudayahu, tagahu, and totaluo from February to September 2022 daily during their appearance season at all the observed locations, as shown in Fig. 1. Furthermore, the collected sample was preserved in an icebox and taken immediately to the Hydrobioecology and Biometrics Laboratory, Faculty of Fisheries and Marine Sciences, Gorontalo State University, for identification. The sample was identified using the method proposed by Sahami et al. (2019, 2020) as well as Sahami & Habibie (2021). Species with new melanophore patterns were then photographed, followed by a description of their morphology, and two specimens from each were taken for molecular analysis.
Approximately ± 20 mg of fish muscle tissue in each genetic sample was extracted using a Blood and Tissue Kit (Qiagen, Hilden, Germany). The extracted DNA samples were subsequently subjected to analysis using polymerase chain reaction (PCR) with the primer pair FishF1 5’-TCAACCAACCACAAAGACATTGGCAC-3’ and FishR1 5’-TAGACTTCTGGGTGGCCAAAGAATCA-3’ (Ward et al., 2005), targeting the cytochrome oxidase subunit 1 (COI) gene. According to Aquilino et al. (2011) and Hebert et al. (2003), COI can distinguish species with vague or similar morphology. The samples were then subjected to amplification through predenaturation at 94°C for 3 minutes, denaturation at 94°C for 30 seconds, primary annealing at 50°C for 30 seconds, elongation at 72°C for 60 seconds and final elongation at 72°C for 2 minutes, with a total of 38 cycles. Finally, the samples were sequenced using the Dideoxy Sanger Termination Method at Genetika Science Jakarta Company.
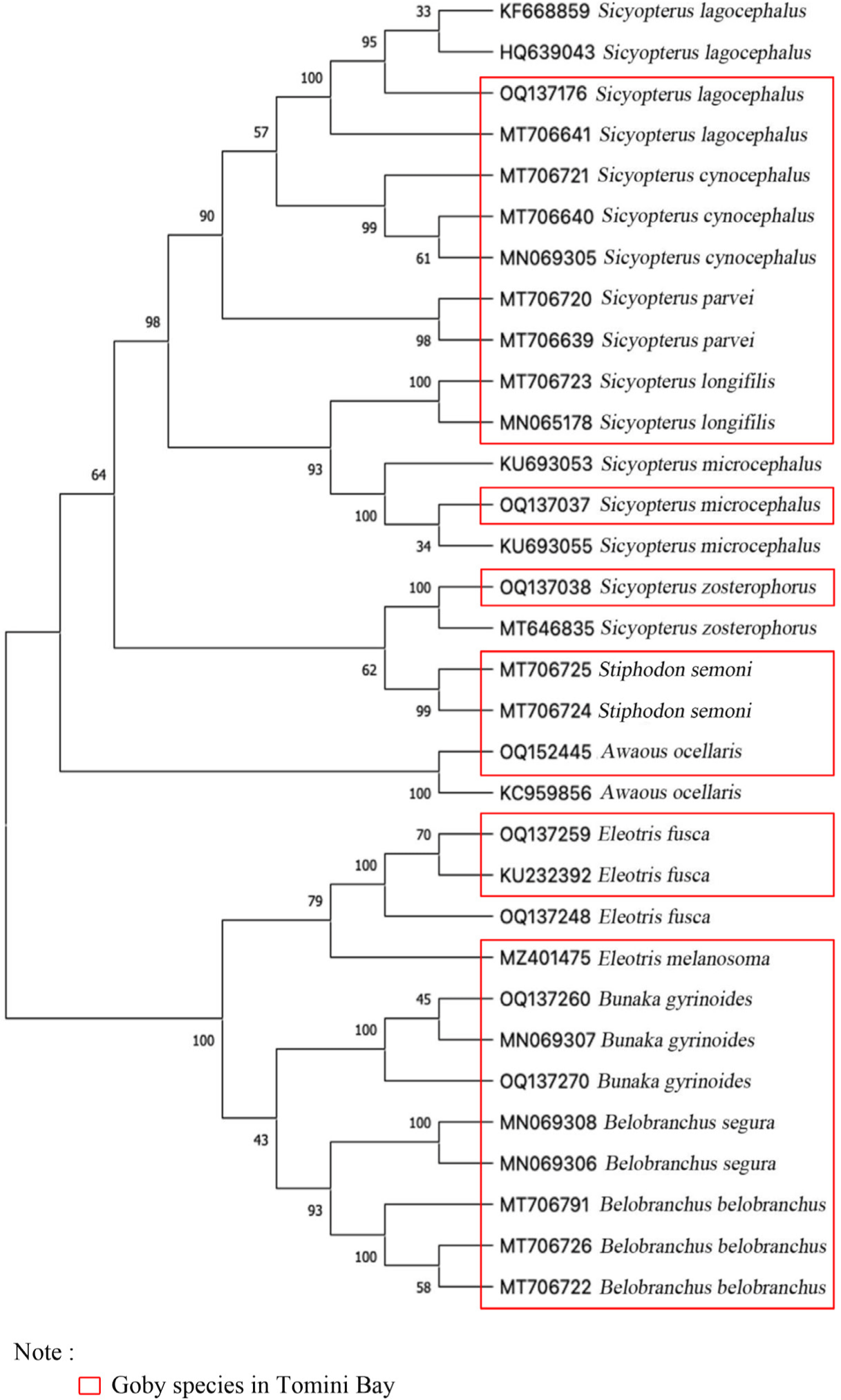
The nucleotide sequences from DNA sequencing processed using CONTIG were matched with the database available at GenBank (https://www.ncbi.nlm.nih.gov) with the Basic Local Alignment Search Tool (BLAST) method. The nucleotide sequences data of the goby-fry and nongoby-fry species identified in this study were deposited into the NCBI database with accession numbers OQ137037, OQ137038, OQ137176, OQ137248, OQ137259, OQ137260, OQ137270, OQ152445, OQ137029, OQ137041, and OQ137043. Subsequently, the goby-fry phylogenetic tree was constructed by aligning the DNA sequences of the sample with those obtained from the GenBank database comprising accession numbers MN065178, MN069305, MN069306, MN069307, MN069308, MT706639, MT706640, MT706641, MT706720, MT706721, MT706722, MT706723, MT706724, MT706725, MT706726, MT706791, MZ401475, HQ639043, KC959856, KF668859, KU232392, KU693053, KU693055, and MT646835, for certain validations. Phylogenetic tree construction was made using the Maximum Likelihood 1000 bootstrap method in MEGAX (Molecular Evolutionary Genetic Analysis, Pennsylvania State University, University Park, PA, USA) software (Kumar et al., 2018).
Results
A total of 75,881 goby-fry and 1,687 non-goby-fry samples were successfully collected from five river estuaries in Tomini Bay, Gorontalo City, Gorontalo Regency, and Bone Bolango Regency. These samples belonged to Eleotridae and Gobiidae of the goby family, and accounted for 10.164% and 87.661% of the fish group. The three main families of non-goby, included in this research are Anguillidae, Moringuidae, and Syngnathidae, which made up 2.166%, 0.003%, and 0.006%. Among the collected goby samples, a total of five and eight species were identified in the Eleotridae, and Gobiidae families, respectively. The distribution of these species across the five observed locations is shown in Table 1.
The study identified three species as constituent goby-fry schools, namely Eleotris fusca, Sicyopterus microcephalus, and Sicyopus zosterophorus. The identification process involved analyzing melanophore patterns and COI DNA gene analysis, as shown in Table 1. Interestingly, the results stated that E. fusca was not found in the Taludaa Estuary, while S. microcephalus was absent in both the Paguyaman and Taludaa Estuaries. S. zosterophorus was distributed across five different fishing locations in Tomini Bay. Furthermore, this study made an exciting discovery of new melanophore patterns for B. gyrinoides, as shown in Fig. 2. This result provided valuable information about the characteristics of this species in the area.
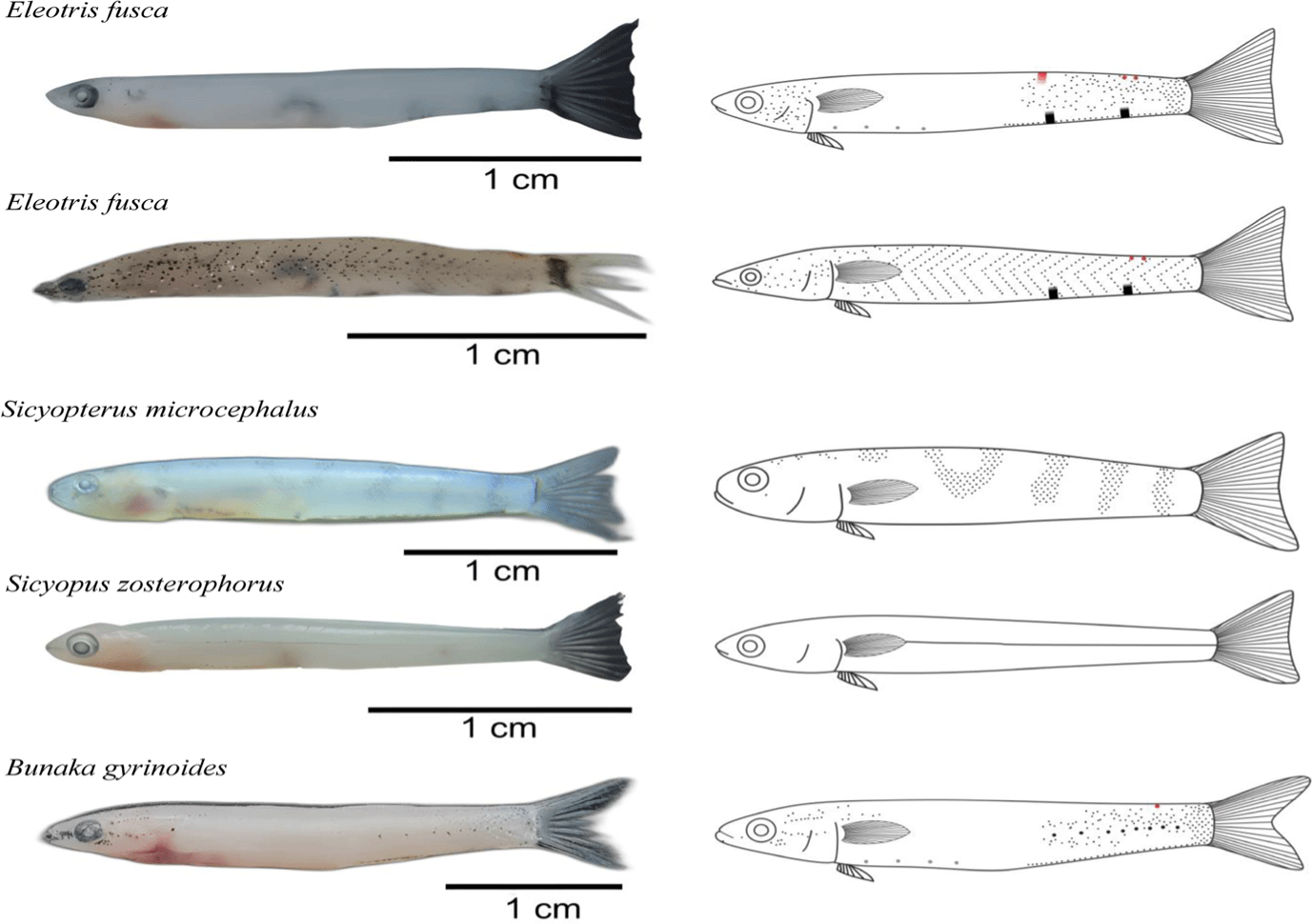
The captured E. fusca specimens have an elongated and compressed body shape, a transparent body, a lack of scales, and an emarginate caudal fin. In E. fusca, this was confirmed through nucleotide sequence analysis, which revealed two distinct melanophore patterns. The first melanophore pattern was depicted by dots stacked on the head, near the base of the tail, and on the ventral side of the body. There are also two thickened melanophores on the ventral side, one erythrophore in the form of a vertical line, and two erythrophore dots on the back near the tail were visible. The second pattern formed an arrow-like shape, extending from the tail to the body parallel to the pectoral fin. This pattern also included two thickened melanophores on the ventral side and two erythrophore dots on the back approaching the tail, similar to the first E. fusca pattern.
S. microcephalus is characterized by an uncompressed shape, a transparent body, a horseshoe-shaped melanophore on its back, a lack of scales, and a caudal fin that tends to form a truncate shape. Meanwhile, S. zosterophorus has an elongated and compressed body shape with a unique melanophore pattern that forms a thin horizontal line along the lateral one. Furthermore, B. gyrinoides shows melanophore dots, which accumulate on the head and body near the base of the tail. There were also thicker dots along the tail, arranged horizontally on the lateral line.
The study conducted in Tomini Bay, Gorontalo, reported a significant discovery on goby-fry species, and nongoby-fry species mixed with schooling goby-fry. Non-goby-fry specimens were grouped into three distinct categories based on their morphological appearance. These three species, namely Anguilla celebesensis, Moringua microchir, and Microphis leiaspis, were observed and captured alongside the goby-fry school within the river body, as shown in Fig. 3. Interestingly, the occurrence of these three nongoby-fry species varies in each location. A. celebesensis was found in 3 out of 5 study locations, namely Bone-Bolango, Bilungala, and Paguyaman Estuary. Furthermore, M. leiaspis was found in the Bone-Bolango and the Paguyaman Estuary, while M. microchir was only found in the Paguyaman Estuary. This observation represents the first documented instance of such a phenomenon in this specific region, adding valuable new information to the understanding of the aquatic ecosystem in Tomini Bay.
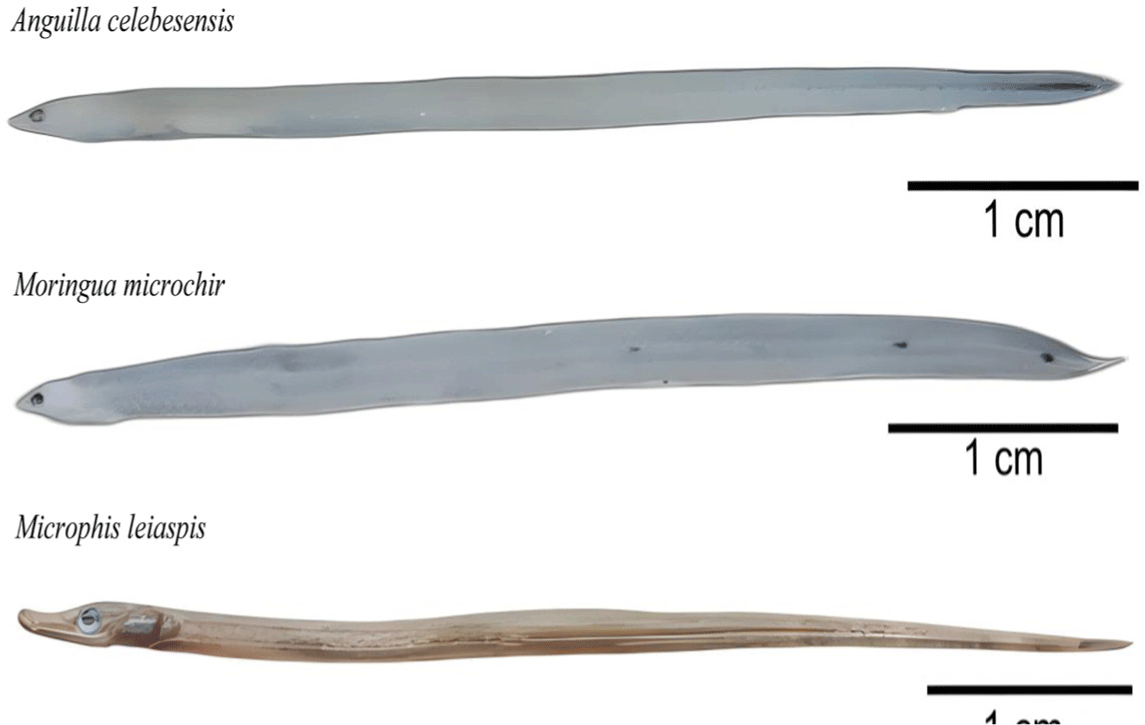
Both A. celebesensis and M. microchir belong to the Anguilliformes group and were captured during the glass eel phase, characterized by their transparent body. However, these are distinguished by A. celebesensis and M. microchir different melanophore patterns. A. celebesensis is characterized by a pattern of melanophores spreading on its tail buds. Whereas M. microchir, also known as spaghetti eels, lacks a melanophore pattern on its tail buds. In terms of appearance, M. microchir has a transparent body with relatively thick melanophore dots arranged at a distance from the linea lateralis. These dots extend from near the tail to the middle of the body. Furthermore, this species is recognized by its long, extremely slender, rigid body structure, and lengthy tubular brownish snout. These distinctive characteristics are essential for identifying and differentiating the two species in the Anguilliformes group during the glass eel phase.
The results of the DNA sequence alignment of the goby-fry species in Tomini Bay, Gorontalo, were presented as a phylogenetic tree in Fig. 4. Furthermore, the analysis revealed two distinct monophyletic clades. The first monophyletic clade of gobies consists of species in the family Gobiidae. It is further divided into subclades, namely S. lagocephalus and S. cynocephalus in the first subclade, S. parvei in the second subclade, S. longifilis and S. microcephalus in the third subclade, S. zosterophorus, and S. semoni in the fourth subclade, as well as A. ocellaris in the fifth subclade. The second monophyletic clade consists of species in the Eleotridae family. This includes E. fusca and E. melanosoma in the first subclade, as well as B. gyrinoides, which have a close relationship with B. belobranchus and B. segura in the second subclade. The phylogenetic tree provides a clear visualization of the evolutionary relationships and genetic similarities among the goby-fry species in Tomini Bay. It offers valuable insights into these species genetic diversity and relatedness within their respective families.
Discussion
Since the initiation of the study on the genetic diversity of goby-fry schools in Gorontalo Bay (Bone-Bolango estuary), a total of eight species have been documented from previous investigations. These species include S. longifilis (Olii et al., 2019; Sahami et al., 2020), S. cynocephalus, B. gyrinoides, and B. segura (Sahami et al., 2019), as well as S. parvei, B. belobranchus, S. semoni, and S. lagocephalus (Sahami et al., 2020). In the waters of Paguyaman Bay (Bilato estuary), seven goby-fry species have been reported, namely S. longifilis, S. parvei, S. cynocephalus, S. semoni, B. segura, B. belobranchus, and E. melanosoma (Sahami & Habibie, 2021). Three new species, namely E. fusca, S. microcephalus, and S. zosterophorus, were identified as constituents of the goby-fry hordes in the waters of Tomini Bay. These results have significantly increased the level of species diversity in these waters. Additionally, this study also provided a first-time report on the distribution of goby-fry schools in the estuaries of the Paguyaman, Bilungala, and Tombulilato Rivers in Tomini Bay, Gorontalo. The existence of rivers that flow into the waters of Tomini Bay, Gorontalo, further clarifies the distribution of amphidromous goby-fry in this region, as shown in Fig. 1. Goby is a fish family with the highest species richness, but limited studies have been carried out on the biological aspects, including genetic diversity (Čekovská et al., 2020), in Indonesian waters. This group of fish holds significant importance as a target for consumption, specifically during the migration back to the river (Nurjirana et al., 2020). The ongoing investigation and discoveries contribute to a better understanding of the intricate dynamics and biodiversity of goby-fry in Gorontalo Bay.
B. gyrinoides pattern previously researched by Sahami et al. (2019), is different from the one in this research. In this study, melanophore dots of B. gyrinoides were piled up close to the head and body near the base of the tail. Thicker dots were also found along the tail, arranged horizontally on the lateral line. Sahami et al. (2019) stated that the melanophore dots found in B. gyrinoides exhibited almost covered the entire body. Its variations are influenced by factors such as the environment, age, and natural dichromatism (Sahami et al., 2020).
The captured E. fusca specimens have almost identical morphological characteristics to E. melanosoma, namely an elongated and compressed shape, a transparent body, a lack of scales, and an emarginate caudal fin (Sahami & Habibie, 2021). However, a distinguishing feature between the two species was the presence of a different melanophore pattern. This study found two distinct melanophore patterns of E. fusca, which confirmed genetically. These distinct melanophore patterns played a relevant role in confirming the species as E. fusca and distinguishing them from closely related species such as E. melanosoma. The results shed light on the unique characteristics and identification of E. fusca in the studied region.
E. fusca, a widely distributed amphidromous species in the Indo-Pacific region, undergoes a life cycle characterized by a pelagic larval phase in the sea, followed by returning to the river for growth and reproduction (Mennesson et al., 2015). Despite its broad distribution, this species has been classified as endangered in Reunion Island (UICN France et al., 2013) due to habitat degradation and hydrological shifts, which have the potential to impede post-hatching emigration and post-larval recruitment immigration (Arthington et al., 2016; Mennesson et al., 2015). These threats pose significant challenges to the survival and population of E. fusca in the area.
S. microcephalus and S. zosterophorus belong to the Gobiidae family. A distinctive feature of S. microcephalus is a horseshoe-shaped melanophore pattern on its back, which is not found in other Sicyopterus species (Sahami et al., 2019, 2020). The species S. microcephalus has been reported to be distributed in the waters of the Luwuk Banggai River (Gani et al., 2019), as well as the sea waters of Lariang Village, both in Sulawesi, Indonesia (Nurjirana et al., 2022a). On the other hand, S. zosterophorus can be found in various locations, ranging from Sumatra (Indonesia) to South Japan, Vanuatu, and New Caledonia (Keith et al., 2015). It is also found in Koyoan, Sulawesi, Indonesia (Gani et al., 2019), eight rivers in the Luwuk Banggai (Gani et al., 2019), and Simpong (Gani et al., 2021). This species inhabits clear, fast-flowing rivers with pebbly water substrates (Keith et al., 2015). Taillebois et al. (2013) conducted spatial analyses of genetic variation in S. zosterophorum, revealing strong isolation across the Torres Strait, which once served as a geologically intermittent land barrier connecting Australia to Papua New Guinea. A clear genetic break was found between the northwestern and the southwestern clusters in S. zosterophorum. These results provide valuable insights into the population structure and genetic diversity of this species across its wide distribution range.
In Gorontalo, goby-fry has been reported to form schools of postlarvae and juveniles from the Gobiidae and Eleotridae family, with a higher dominance of the Gobiidae family (Olii et al., 2019; Sahami & Habibie, 2021; Sahami et al., 2019, 2020). Similarly, the dominance of the Gobiidae family over the postlarvae group was also found in West Sulawesi waters (Nurjirana et al., 2020). Several nongoby-fry species, namely A. celebesensis, M. microchir, and M. leiaspis, were reported for the first time in this study as schooling groups along with goby-fry during migration from the sea waters of Tomini Bay, Gorontalo to estuaries. In the waters of West Sulawesi, large numbers of migrating goby postlarvae were occasionally accompanied by eel larvae (Nurjirana et al., 2020). However, specific eel species and their morphological characteristics are yet to be reported. Eel larvae have significant economic value to fishermen, making this study relevant for understanding their presence and ecological impact. This study provides valuable insights into the local aquatic biodiversity and ecosystem dynamics by uncovering the dynamic interactions between goby-fry and eel larvae populations.
A. celebesensis was found in the West Pacific Ocean, spanning from Indonesia to the Southern Philippines, with a prominent presence in the Celebes Sea and Tomini Bay (UNEP-WCMC, 2015). This fish has a catadromous migration pattern, with the adult individuals spawning in seawater, while the hatched eggs develop into leptocephalus and eventually metamorphose into glass eels. The samples then migrate to fresh waters, where they continue to grow into yellow and silver eels. Once mature, these eels return to the sea to spawn again (Arai et al., 2003). Eels usually undergoe significant morphological changes in its planktonic phase, namely leptocephalus. Despite maintaining a round and elongated body shape, the color and size exhibit variations (Ndobe et al., 2019). A visible backbone characterizes the glass eel phase because the body is still transparent. Melanophores also form on the head and tail, with variations observed between species (Silvfergrip, 2009). This pattern distinguishes A. celebesensis from A. japonica, and they both lack melanophores on the fins and caudal buds, as well as Anguilla bicolor pacifica, which bears markings solely on the caudal fin (Leander et al., 2012). Although Leander et al. (2012) reported that A. celebesensis, Anguilla luzonensis, and Anguilla marmorata have similar melanophore pattern in their tail buds, the species identification were further strengthened through genetic analysis of the DNA, which identified these species as A. celebesensis. The combination of morphological and genetic analyses provides a robust and reliable method for accurate species identification and classification.
Several studies consistently reported that the spawning locations of A. celebesensis are in the high seas of Sulawesi and Tomini Bay, Central Sulawesi (Aoyama et al., 2003). This result is in line with this present study, confirming the distribution of this species in the estuaries of the Tomini Bay area of Gorontalo, including Bone-Bolango, Gorontalo City; Bilungala, Bone Bolango Regency; and Paguyaman estuary, Gorontalo Regency. A. celebesensis was reported to be distributed in various locations, such as the Poso and Poigar Rivers, Sulawesi, Cagayan River, Philippines (Arai et al., 2003), Palu estuary (Ndobe et al., 2019), Lake Tondano (Kottelat et al., 2013), as well as Poso Rivers and Lakes (Hagihara et al., 2018). However, this species were not reported in Tomini Bay, Gorontalo. Hewavitharane et al. (2018) stated that A. celebesensis recruitment occur yearly, and migration downstream typically starts from the beginning to the middle of the rainy season, as observed in the waters of Lake Poso, Sulawesi, Indonesia (Hagihara et al., 2018). This species was also included in the Near Threatened (NT) category based on the 2014 IUCN classification (UNEP-WCMC, 2015).
M. microchir is distinguished by its elongated and tapered body shape complemented by small pectoral fins. The juveniles have an orange-yellow back and bluish belly, while the adults have a brown color on their backs and a lighter belly (Eudeline, 2022). Furthermore, the distribution of M. microchir spans various regions, from South and East Africa, Seychelles, Madagascar, and East Mauritius to Samoa, the north of the Ryukyu Islands, the south of the Great Barrier Reef (Australia), and New Caledonia (Fricke et al., 2018). This species has also been found in Ambon and Sumatra, Indonesia (Kottelat et al., 2013). Despite these records, information on the distribution of M. microchir in Sulawesi waters remains limited (Miesen et al., 2016). These species have also been observed in the waters of the Kolobangara River, a priority site on Choiseul Island (Keith et al., 2015, 2016, 2021). M. leiaspis exhibits an amphidromous migration pattern (Ishihara & Tachihara, 2008) such as a goby-fry fish, where larvae that have just hatched in river waters experience a phase of life in the sea. During the postlarvae stage, they return to river waters for growth and reproduction. This amphidromous migration pattern adds an interesting dimension to the life cycle of the species and contributes to the intriguing ecological dynamics surrounding M. leiaspis.
Effective habitat conservation at the local scale should be complemented by broader management strategies that consider the distribution of organisms (Frotté et al., 2019). Understanding the distribution patterns, basic topology, and local physical environment serve as a critical framework for marine zoogeography (Murase et al., 2017). The results of this study implied that among the 13 species of gobi-fry, seven of them were distributed across the river estuaries in Tomini Bay, Gorontalo. The remaining six species, namely A. ocellaris, E. melanosoma, B. gyrinoides, S. microcephalus, E. fusca, and S. parvei, were not found in all the study locations. In addition, an interesting occurrence of nongoby-fry schooling and goby-fry species, specifically A. celebesensis, M. microchir, and M. leiaspis, was also discovered. This study marks the initiation of molecular reference libraries for goby-fry fish in Tomini Bay, Gorontalo. Enhancing these libraries requires further exploration of other fishing locations. Comparing recruitment, understanding associated species, and comprehending the biological cycle of amphidromous species are vital steps to manage and conserve these organisms for sustainable fishery effectively. Such comprehensive efforts are essential to promote conservation practices and responsible management in the region.