Introduction
The Natuna Sea is a fishing ground potential for vessels located on the borders of Indonesia with Malaysia, Singapore, Vietnam, and China. These waters often become the destination of illegal fishing by foreign fleets because they have high economic fish resources. The pelagic fish in these waters are caught by various fishing gear, including purse seines. Purse seine fishing gear is developing for offshore fishing in almost all waters of the Sunda Shelf, from the waters of Pejantan Island and the Natuna Islands (southern part of the Natuna Sea) to around Balikpapan waters (western part of the Makassar Strait) including the fishermen from West Kalimantan, especially in Pemangkat since 1990 (Atmaja & Sadhotomo, 2000). Initially, the number of purse seine in Pemangkat waters around 1995 was only six units, but over time, it increased to 48 units in 2003 (Hariati et al., 2009).
Mackerel fish dominate the catch composition of the purse seine fishery in Natuna Sea waters (Decapterus spp.) as much as 67.3%, followed by spotted sardinella (Amblygaster sirm) with 6.5%, Indian mackerel (Rasterlliger kanagurta) with 6.1% respectively, Sardines (Sardinella spp.) by 5.4% and Bigeye scad fish (Selar crumenophthalmus by 3.6%) (Atmaja & Nugroho, 2004). Although spotted sardinella is not the main catch but this fish is one of the targets for catching in general. The public widely consumes this fish because it has a very high fatty acid content, which can potentially become omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are suitable for human health (Suseno et al., 2014). The high consumption demand for spotted sardinella will spur an increase in resource capture efforts using purse seines.
The spotted sardinella are essential to the population as they are part of the large pelagic fish food chain (Kasim et al., 2014). It means that the exploitation status of spotted sardinella can also affect other fish populations because they are in the same food chain. Spotted sardinella preys on tuna in Pemangkat waters and its surroundings (Hidayat, 2022). It is also the raw material for the fish canning industry in some areas, making it a vital commodity (Purwoko & Prasetyo, 2019). It shows that spotted sardinella resource has a significant role in various aspects.
Although small pelagic fisheries were developed in the Natuna Sea several years ago, the stock status of spotted sardinella has yet to be reported. A length-based assessment was performed to assess the population dynamics and the stock status of spotted sardinella fisheries in the Natuna waters. This study is expected to contribute to the fisheries management of small pelagic resources in the Natuna waters.
Materials and Methods
The length measurement activities of spotted sardinella were conducted from March 2019 to December 2020 at the Pemangkat landing site (1° 10’ 31.20” N and 108° 58’ 13.43” E), West Kalimantan, Indonesia. The spotted sardinella from purse seine vessels with fishing grounds in the Natuna Sea and Karimata Strait were measured (Fig. 1).
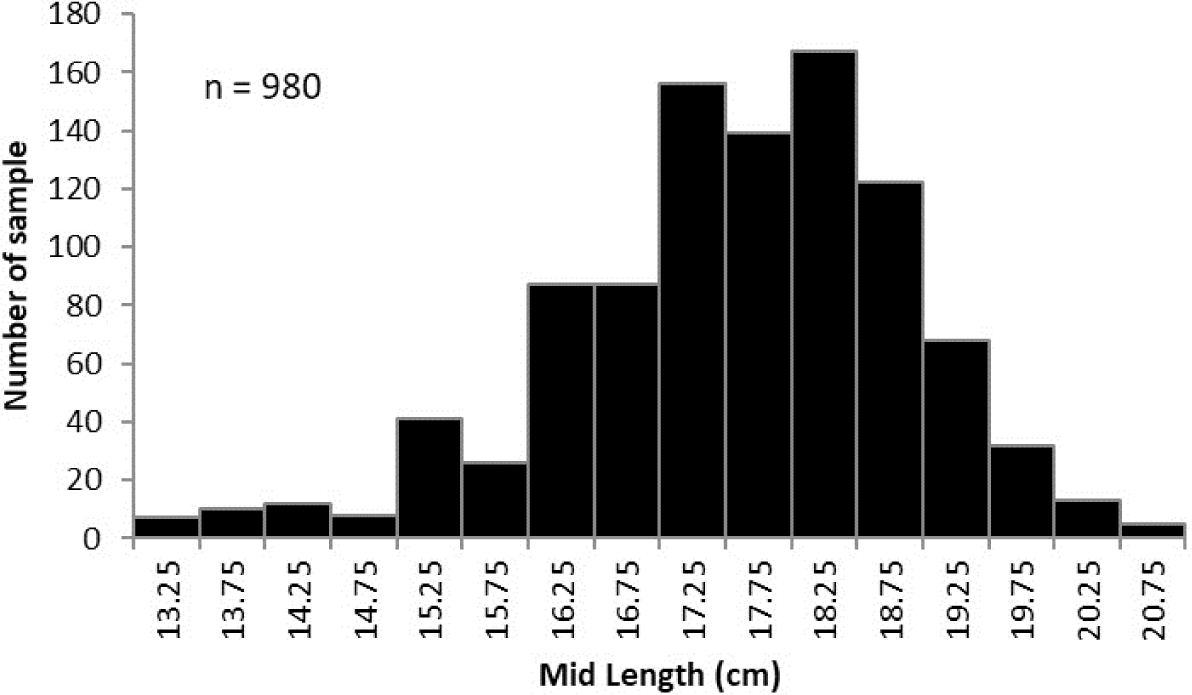
A total of 980 spotted sardinella (Table 1) were measured and weighed. Fork length (FL) is determined to the nearest centimeter (cm) using a vernier caliper. The total weight was taken with an electronic balance to the accuracy of 0.01 g. Data analysis was carried out by tabulating the frequency data of spotted sardinella and grouping them into 0.5 cm length classes. These length-frequency data were used to determine population parameters of spotted sardinella, including cohort identification, growth parameters, mortality parameters, and exploitation rates; length-frequency data were analyzed using FAO-ICLARM Stock Assessment Tools (FiSAT) software (Gayanilo et al., 1996). The length-weight relationship is obtained using the exponential equation W = aLb (Ricker, 1975). Sparre & Venema (1998) estimated length at first capture (Lc50). The logistic function equation was used to estimate length at first maturity (Lm50) (King, 2007).
| Area | K | L∞ | Reference |
|---|---|---|---|
| Natuna Sea, Indonesia | 0.85 | 26 | Atmaja & Nugroho (2004) |
| Andaman Water, India | 0.77 | 27.4 | Pradeep et al. (2014) |
| Sunda Strait, Indonesia | 0.46 | 23.6 | Kartini et al. (2017) |
| Java Sea, Indonesia | 0.95 | 20.5 | Sari et al. (2021) |
| Red Sea, Egypt | 0.35 | 27.4 | Osman et al. (2021) |
The age group (cohort) of spotted sardinella was determined using the logarithmic difference method by Bhattacharya from the Package FiSAT (Gayanilo et al., 1996). This method separates several normal distributions, each representing a cohort of fish, from the overall distribution, starting from the left-hand side of the total distribution of fish divided into length classes.
Von Bertalanffy’s growth function (VBGF), which was determined by Sparre & Venema (1998), is built using the asymptotic length (L∞), growth coefficient (K), and time (t0) parameters. Equation Pauly (1983) is used to get the t0 value of the spotted sardinella. Based on Zhang & Megrey (2011), the natural mortality (M) was calculated, and using the FiSAT program, total mortality (Z) was determined (Gayanilo et al., 1996). Pauly (1983) is the basis for estimating fishing mortality (F). The exploitation rate (E) was determined using the formula given the values of F and Z (Gulland, 1971).
The spawning potential ratio (SPR) can be used to estimate the exploitation status by using a length data-based (length-based SPR) (Hordyk et al., 2015). The SPR value will be high if large/adult fish dominate the caught, and conversely, the SPR is low if the small fish are caught in large numbers (Hordyk et al., 2015; Prince et al., 2014). The SPR value will be high if the catch is predominately made up of large/adult fish and low if the catch is mainly made up of little fish (Hordyk et al., 2015; Prince et al., 2015). The SPR was determined by the comparison of the spawning potential in the presence of fishing (SSBRfished) and the spawning potential in the absence of fishing mortality (SSBRunfished) based on the equation of Goodyear (1993).
Results
Spotted sardinella caught by Purse Seine in Natuna waters ranged in length from 13–20.9 cm with an average of 17.78 ± 3.40 (mean ± SD). The mode size was 18–18.5 cm, with a proportion of 17% (Fig. 2).
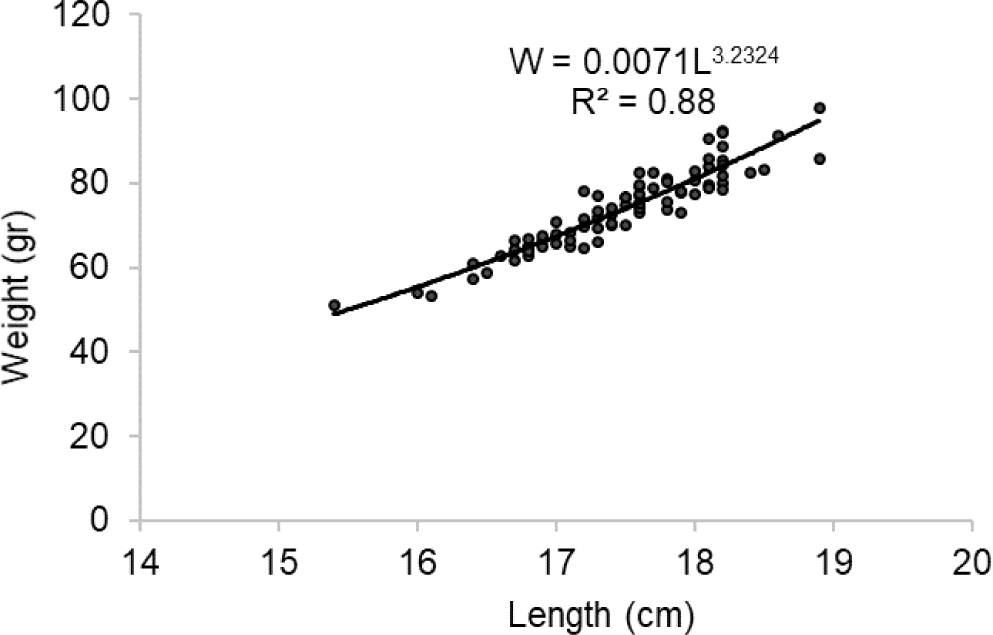
The length-weight relationship of spotted sardinella was estimated as W = 0.0071L3.2324, and the b value is 3.2324, indicating a positive allometry pattern (Fig. 3). It shows spotted sardinella have faster body weight gain compared to length growth.
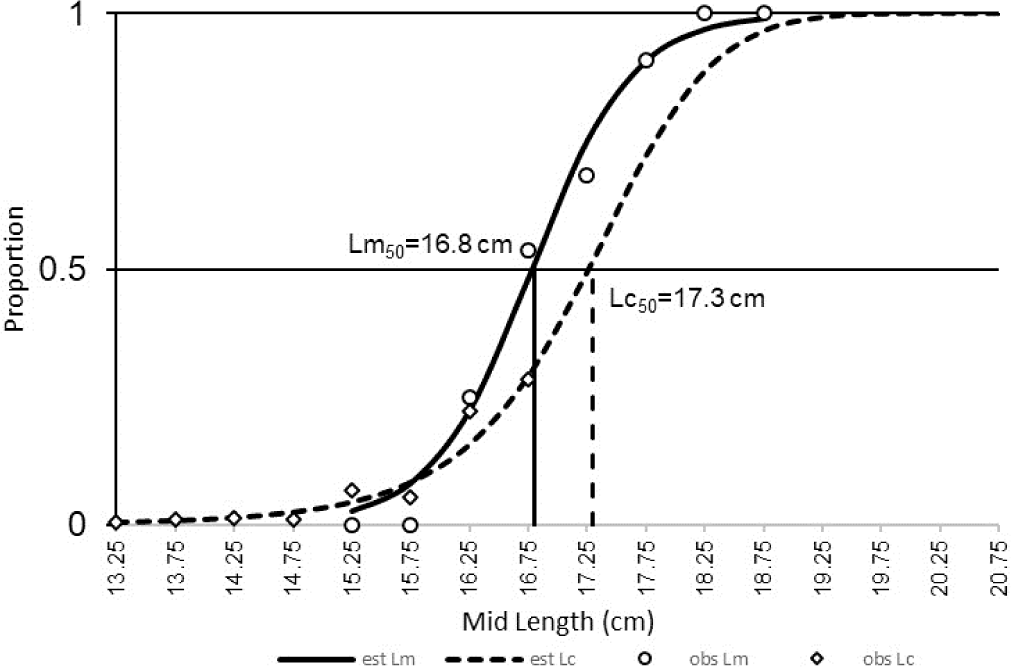
In this study, the Lc50 of spotted sardinella (17.3 cm) was more significant than the Lm50 (16.8 cm) (Fig. 4). These conditions indicate that most of the spotted sardinella caught have spawned.
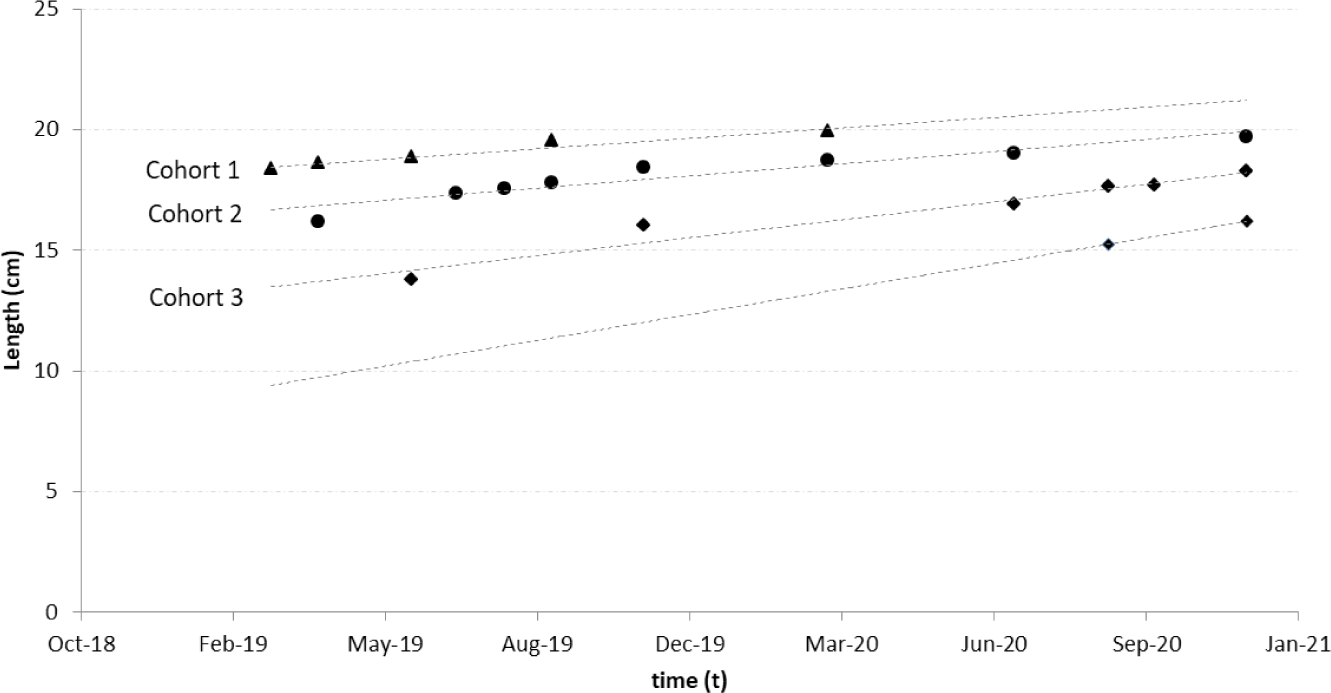
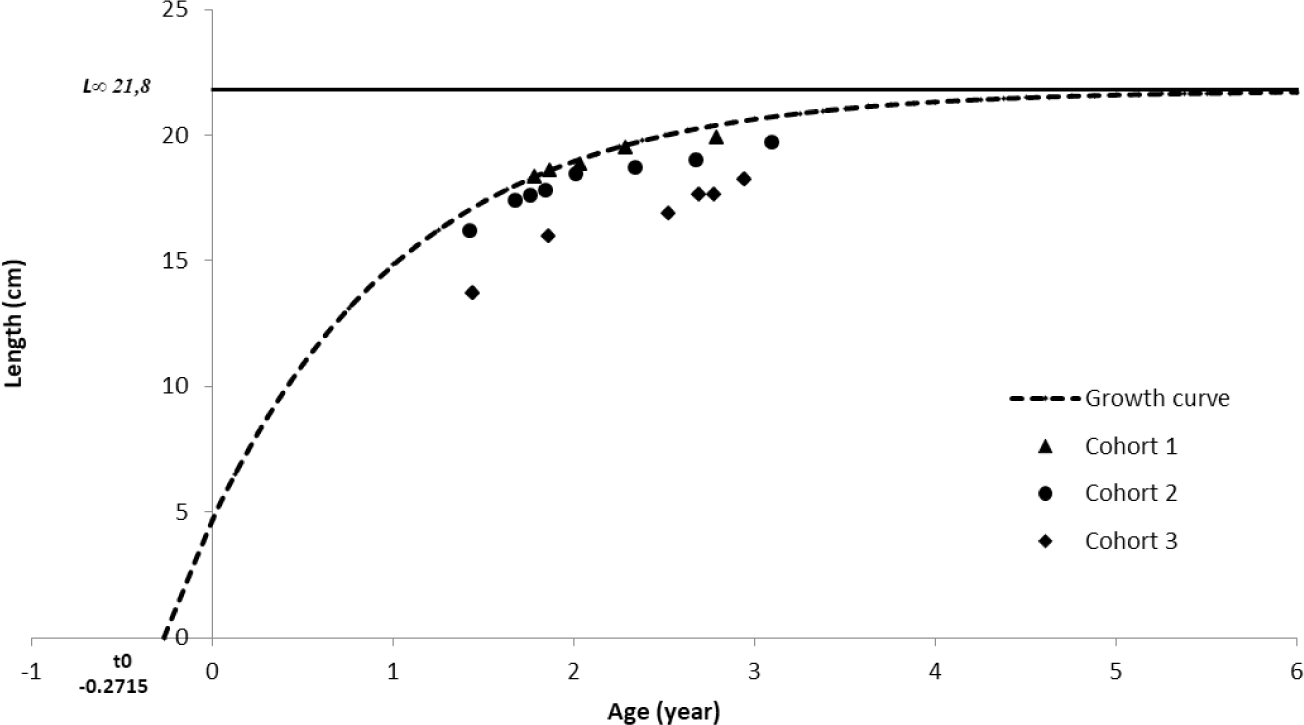
Age group analysis showed three primary cohorts of spotted sardinella in the Natuna Sea. The first cohort appeared at the beginning of the observation (February 2019) at 18.3 cm and was the oldest cohort recorded. It last appeared in March 2020 at 19.9 cm. The second cohort was the most frequent. This cohort was recorded from April 2019 to the end of the study period in December 2020. The second cohort was younger than the first cohort. The youngest cohort emerged in June 2019 with a length of 13.7 cm and continued to grow until the end of the observation month, December 2020. A new cohort (cohort zero), as the initial recruitment, reappeared in September 2020 at 15.3 cm (Fig. 5).
Based on the b value of 3.23, growth rate (K) of 0.65 year−1, Ci constant for small pelagics of 0.3, lifespan (tmax) of 4.3 year, and t0 of –0.27146 year, the value of the fishing mortality rate (M) is 1.19 year−1. Calculation of the total mortality was carried out by using some input parameters L∞ (21.79 cm), K (0.65 year−1), and t0 (–0.27146 year) to obtain the relationship between age (x-axis) and ln(N/dt) (Fig. 6). The peak of the regression is used as the starting point in determining the slope value which is the value of the total mortality (Z) of 2.19 year−1. The capture mortality rate (F) was found to be 0.99 year−1 from the two mortality rate values. The exploitation rate (E) of spotted sardinella in the Natuna Sea is obtained by the ratio of the fishing mortality rate (F) and the natural mortality rate (M), that resulting of 0.45.
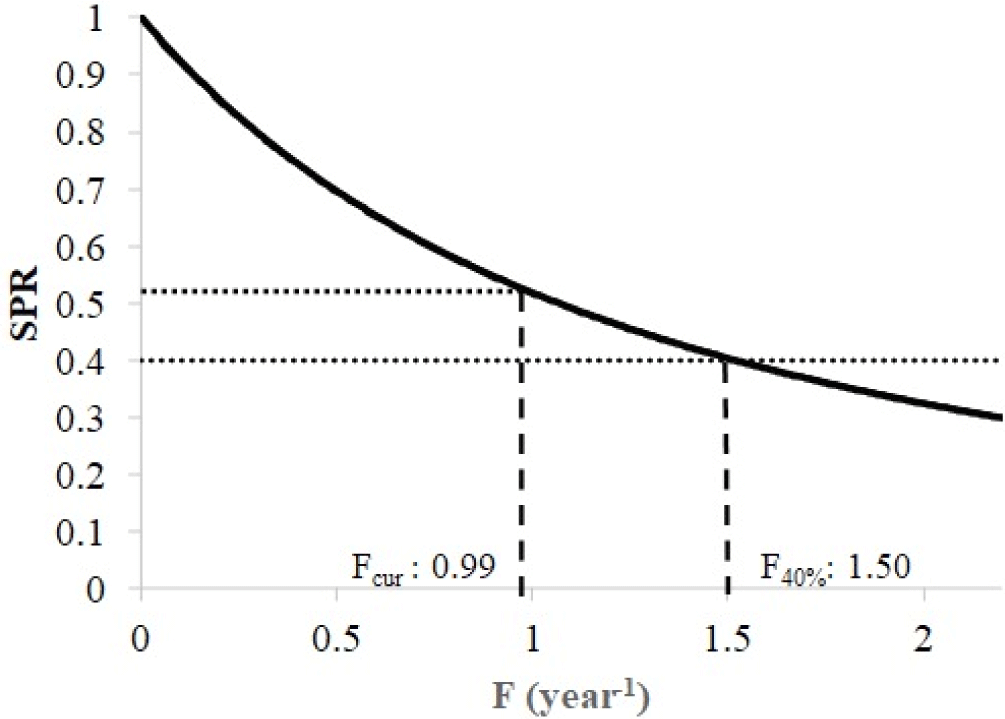
A length-based SPR of spotted sardinella in the Natuna Sea has been performed by using some input parameters, including the asymptotic length (L∞) 21.79 cm, growth rate (K) 0.65 year−1, natural mortality (M) as 1.19 year−1, the length at 50% selectivity (Lc50) 17.3 cm, the length at 95% selectivity (Lc95) 20 cm, the length at 50% maturity (Lm50) 16.8 cm and the length at 95% maturity (Lm95) 20 cm. A population of spotted sardinella in the Natuna Sea has a SPR of 52%. This condition indicates that 52% of The remaining fish population in nature still has the potential to spawn. Fishing mortality (Fcur = 0.99) is currently lower than the target reference point (F40% = 1.5), showing that the spotted sardinella stock is still sustainable (Fig. 7).
Discussion
Spotted sardinella in this study ranged in size from 13 to 20.9 cm, while Pradeep et al. (2014) in the Andaman Sea revealed that loungers ranged from 18.6 to 26 cm (total length), in the Java sea from 12 to 20.5 cm (total length) (Sari et al., 2021), and in the Sunda Strait is between 10 and 18.9 cm (FL) (Kartini et al., 2017). Spotted sardinella has a length of 12.3 to 24 cm (total length) in the Red Sea off the coast of Egypt (Osman et al., 2021). The length frequency might vary between regions due to the availability of prey, environment, fishing ground, and fishing season (Mardlijah et al., 2022).
Furthermore, the length-weight relationship indicates the growth pattern of fish (Ragheb, 2023). The length-weight relationship of spotted sardinella showed a positive allometric condition consistent with that found in the Red Sea (Osman et al., 2021). The b value is also an essential part of growth as it is affected by food availability and seasonality (Jisr et al., 2018), so spotted sardinella in these waters have fatter bodies. The length-weight relationship was influenced by several factors, including environment, diet, sex, gonadal maturity, and gut condition at capture time (Pane et al., 2021).
The condition of Lc50, more significant than the Lm50, is optimal for letting some fish spawn. However, in general, the spawning state of spotted sardinella is partial (partial spawning) based on variations in the gonad maturity index according to the size and level of gonad maturity (Widiyastuti & Zamroni, 2017). The Lc50 and the Lm50 are related to the same fish sample measured, fishing area, mesh size, and growth of male and female fish caught (Mardlijah et al., 2022). Therefore, this should be a concern so that the fish caught are above 17 cm and are closely related to the mesh size used in purse seine gear.
The Lm50 of spotted sardinella in Natuna Sea waters is the same as the Lm50 in the Java Sea, which is 16.8 cm (Purwoko & Prasetyo, 2019). The Lm50 of female spotted sardinella in the Sunda Strait was 18.6 cm (Kartini et al., 2017), while in Tanzanian waters was 16.92 cm (Sululu et al., 2020). The Lm50 of this species appears to vary in several locations due to differences in geographical conditions and fishing pressure (Haig et al., 2015). The Lc50 value of the purse seine is greater than that of Lm50. This indicates that most spotted sardinella caught by the purse seine were adults. They have spawned at least once before being caught. The selectivity of purse seine fishing gear in the Natuna Sea is still quite optimal for spotted sardinella fishing, where most of the fish caught have matured gonads.
Cohorts or “broods” are groups of individual fish of the same species originating from the exact birth (spawning) (Sparre & Venema, 1998). As well as being a basis for age studies, cohort identification also serves as a comparison in stock studies. This is considering that pelagic fish stocks are dynamically changing in the natural world. According to Suwarso & Hariati (2002) sardinella fish only have one primary cohort per year in the Java Sea. Still, Indian mackerel (R. kanagurta) and shortfin scad (Decapterus macrosoma) also have one primary cohort because they are closely related to sardinella fish in reproduction. This study shows that spotted sardinella has several cohorts in the population; it is suspected that the recruitment pattern will have more than one peak (multiple spawning). In addition, the distribution of sizes in each month of observation dramatically influences the appearance of the cohort components. The Natuna Sea’s purse seine fishery catches spotted sardinella between 1.4 and 3.1 years old (at length 13–21 cm). Spotted sardinella collected in the smallest size seemed to have outgrown its juvenile stage (fingerling). Spotted sardinella is thought to have a life span (longevity) of up to 4.3 years.
Growth rate (K) 0.65 This shows that spotted sardinella has a fast growth rate (Sparre & Venema, 1998). Infinity length (L) and growth rate (K) values differ from the findings of other investigations (Table 1).
Differences in growth rates are influenced by internal factors (such as genetics and physiology) and external factors. Table 1 some K values are below 0.5, and some are above 0.5, which is thought to be due to differences in sample size and fishing gear. The values of growth rates can also vary depending on various habitats, including population density, water temperature, and prey availability (Ju et al., 2016). The total mortality parameters for spotted sardinella in the Natuna Sea (Z), natural (M), and fishing (F) are 2.19, 1.19, and 0.99 year−1, respectively. This value is lower than in the study of this species in the Java Sea, which reached 1.7 year−1 (Sari et al., 2021). Analysis of this species in the Natuna Sea several years ago shows total mortality (Z), natural mortality (M), and fishing mortality (F), which are 2.35, 1.4, and 0.32 year−1, respectively (Atmaja & Nugroho, 2004). Spotted sardinella mortality parameters in the Red Sea such as total mortality (Z), natural mortality (M), and fishing mortality (F), which are each measured at 0.84, 0.63, and 0.12 year−1, respectively (Osman et al., 2021). This species’s mortality parameter has a more excellent value in the Java Sea, where Z, M, and F values are, respectively, 2.71, 1.01, and 1.7 year−1 (Sari et al., 2021). The different size distribution of fish causes the difference in fishing mortality (F) due to the habit of pelagic fish dispersing in schooling (Zamroni et al., 2019). The various natural mortality is influenced by several factors: fishing pressure, seawater temperature during sample times, infections, the presence of predators, stress, and old age (Bergström et al., 2022; Sparre & Venema, 1998).
The current spawning ratio for spotted sardinella in the Natuna Sea is 52%. Current fishing mortality (Fcur) = 0.99, which is 36% lower than the target reference point F40% = 1.5. This circumstance demonstrates that the spawning stock biomass in Natuna waters is still relatively ideal for creating new stocks through reproduction. This indicates that spotted sardinella fisheries can continue to be developed because the stock of Natuna Sea’s waters’ exploitation status is still sustainable. However, the development of the spotted sardinella fishery still needs to consider the characteristic conditions of the Natuna water ecosystem. The Purse seine fishery does not selectively catch spotted sardinella but also various other economical species such as scad (Decapetrus spp.), Indian mackerel (R. kanagurta), and bigeye scad (Selar crumenopthalmus). In multi-species fisheries, management can focus on the most vulnerable species or species with the lowest spawning stock biomass (Tirtadanu et al., 2022). Alternatively, fisheries development could focus on other gears that can selectively catch spotted sardinella. In addition, fisheries development needs to be combined with fishing quotas and spawning habitat protection so that the sustainability of the fishery can be maintained.
Conclusion
This study revealed that the fisheries of spotted sardinella Amblygaster sirm is still in the sustainable condition based on the exploitation rate of 0.45 and spawning potential ratio of 0.52. Therefore, the sardinella fisheries can be developed using a precautionary approach and focus on the ecosystem sustainability of FMA 711.