Introduction
Varicorhinus beso —an African scarping feeder fish notable for consuming a range of aquatic invertebrates—is commonly distributed in Ethiopia’s Lake Tana basins (Awoke et al., 2015; Gebremedhin et al., 2012; Mengistu et al., 2017). This fish is used by people in Ethiopia as one of the species with the highest economic value for their food security and income, notably in the Geray reservoir, which is the study’s area of interest. This fish is used by people in the Geray reservoir —the study’s area of interest —as one of the species with the highest economic value for their food security and income (Dzerzhinskii et al., 2007; Mohammed et al., 2016).
Ethiopia’s reservoir fisheries are in jeopardy, notably in the Geray reservoir, as a result of environmental changes brought on by human activities like overfishing and natural disasters like drought (Anteneh et al., 2023b; Anteneh et al., 2023a; Hebano & Wake, 2020). Fisheries management adopts a strategy that satisfies society’s food needs without depleting fish supplies by taking into account the economic, social, and biological elements that have an impact on fish stocks (Garcia & Cochrane, 2005). Fish’s biological studies are essential tools for management and investigation (Shabir et al., 2023; Tesfaye & Wolff, 2015). A close examination of biological characteristics like length-weight relationships (LWRs), condition factors, fecundity, and spawning seasons in a specific fish species is necessary to comprehend the effects of environmental factors, habitat changes, species interactions, and food availability in the ecosystem (Muluye et al., 2016).
The Geray reservoir in the Gulf of Lake Tana basin is home to some different fish species. V. beso is one of the top contenders for the reservoir’s commercial fish output (Mohammed et al., 2016). Nevertheless, due to selective exploitation, the fish population is declining. Therefore, establishing fisheries management plans is essential before the fish are all gone. A successful fisheries management plan requires a thorough understanding of the biological characteristics of a particular fish species in its environment. The relevant morphometric and reproductive traits of any fish species noted in the Geray reservoir are unknown. The goal of this research was to evaluate some biological traits of the African scarping fish V. beso in the Geray Reservoir to develop a successful fisheries management strategy for the habitat.
Materials and Methods
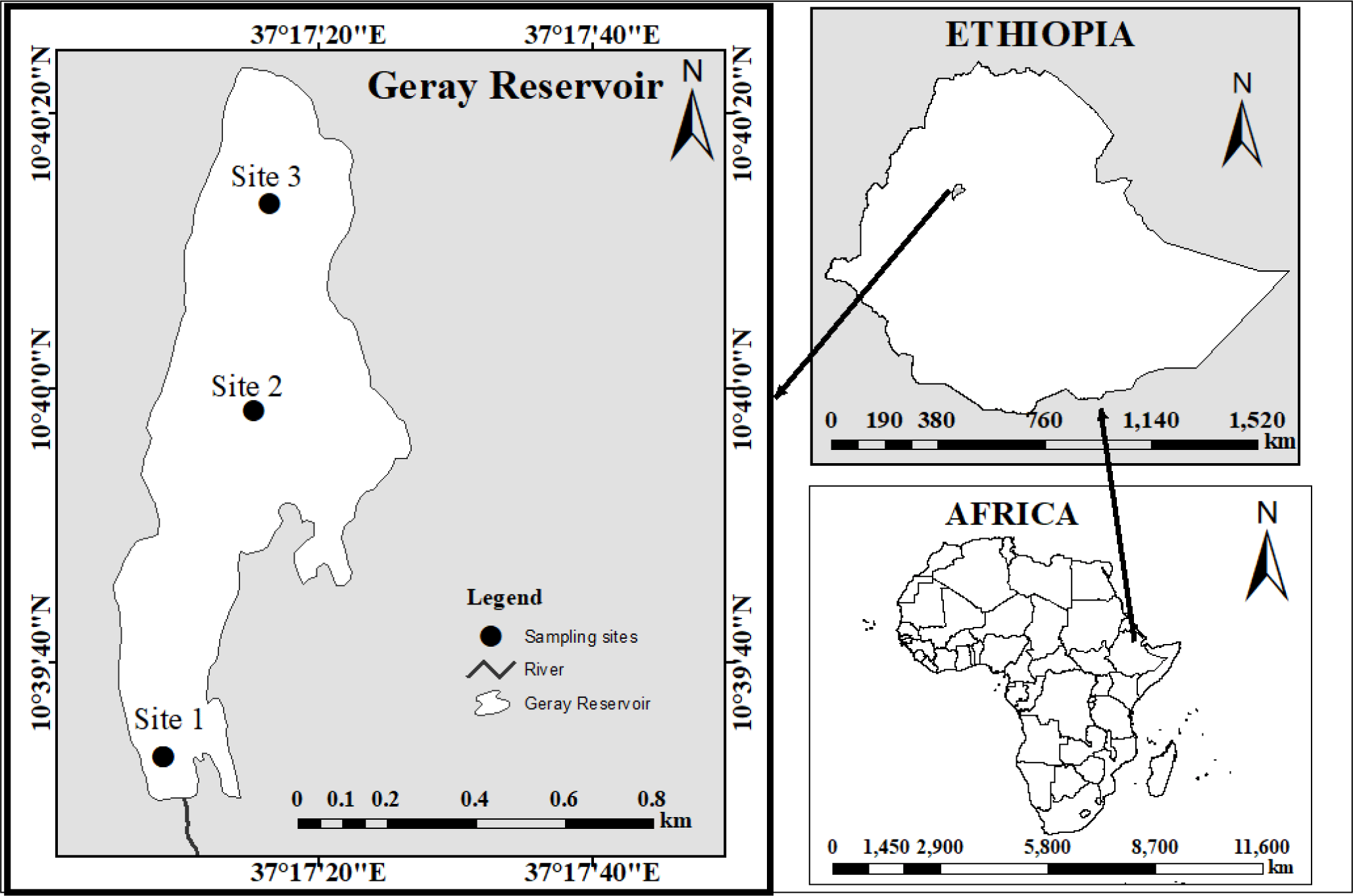
The study was conducted at the Geray Reservoir in Ethiopia. It is 387 and 180 kilometers away from Addis Ababa, the capital of Ethiopia, and Bahir Dar, the capital of the Amhara National Regional State, respectively. It is situated at 37’26” longitude and 10’60” latitude (Fig. 1). The reservoir was constructed for irrigation needs (Checkol & Alamirew, 2008). The Geray Irrigation System is the largest investigated (618 hectares) system illustrative of mid-altitude humid climatic conditions in the Amhara regional state of Ethiopia.
The Geray reservoir has a surface area and volume of 10 ha and 106 m3, respectively. The typical annual temperature and precipitation at Grey Reservoir are 25.75°C and 1,350 mm, respectively (Tegegnie, 2015). The reservoir is home to various species of fish. The Common Carp (Cyprinus carpio), Beso (Varicorhinus beso), Golden fish (Carasius carasius), and Tilapia (Oreochromis niloticus and Tilapia randelli) are the four fish species that are most frequently seen in the Geray reservoir (Mohammed et al., 2016).
This reconnaissance assessment indicated that the wetland is occasionally encroached upon as a result of agricultural activities losing their natural capacity for filtering and buffering, which raises the silt load from the poorly protected watershed. The sediment loads affect the reservoir’s morphology and age, which in turn affects the fish communities in the reservoir, including V. beso, the focus of this study.
Fish samples were taken between October 2022 and May of 2022 from three landing sites on a bimonthly frequency. The sampling sites were characterized based on the level of impairment as highly impaired (inlet site – site 1), less impacted site (pelagic /middle site – site 2), and a moderate level of impacted (outlet site –site 3) (Fig. 1). Fish were collected using cast nets with a mesh size of 6, 8, 10, and 12 cm. Because the fish is dangerously overfished, we employed various mesh-size nets to gather fish of all sizes without using selectivity to obtain a sufficient sample size (fish).
The total length (TL in cm) and total weight (TW in gram (g)) of each fish specimen were measured using a normal ruler (48″ model hinges at 24″) verified against a Vernier caliper to the nearest 0.1 cm and a sensitive digital balance (Model CY510, Citizen, Tokyo, Japan) with a 0.01 g precision, respectively. The LWR equation W = aLb was used to estimate the relationship between the weight (g) of the fish and its TL (cm) (Bolarinwa & Popoola, 2013). The sex of the fish species was determined using the approach recommended by Peña-Mendoza et al. (2005). The fecundity (number of eggs) in the matured female fish was estimated using a gravimetric approach (Bagenal, 1978). The relationship between fecundity and TL and weight of the fish and their gonad weight (GW) was determined using least squares regression. The spawning season was determined using monthly gonadosomatic index (GSI) changes and the percentage of fish with ripe gonads by Hossain et al. (2017). The well-being of V. beso was determined by using the Fulton condition factor (FCF) (Bagenal & Tesch, 1978). The fish captured using various mesh sizes were subjected to the same analysis.
Data analysis was done using IBM SPSS Statistics 20. The statistical significance of the regression model, LWRs, was evaluated using ANOVA. The isometric growth (b = 3) and b predictions for the fish were compared to see if they were statistically substantially different from one another. The significance of the sex ratio and the condition factor were compared between the sexes using the x2-test. The effectiveness of a linear regression model’s result predictions was measured using the coefficient of determination (r).
Results
In the study, a total of 170 fish samples ranging from 21 to 40.2 cm in TL and 94.6 to 618.4 g in TW were collected throughout the collection periods from all sampling sites. When it came to mesh size, there was a significant difference across sampling sites (p < 0.05). Using 10 cm and 12 cm mesh size nets, the largest number of fish samples (n = 122, approximately 70%) were taken at site 3, where there was fairly good macrophyte coverage alongside the watershed. The study demonstrated that in the Geray reservoir, V. beso females’ outnumbered males. 60% of the fish that were gathered were female, and 40% were male. Across the sampling months, the sex ratio fluctuated considerably (t-test; p < 0.05). In December (1:1.3) and March (1:4), females significantly outnumbered males (p < 0.01). Almost equal proportions of males and females were observed in the rest of the sampling months. The overall sex ratio of males to females (1:1.5) of the population when tested statistically showed a significant difference (x2 = 6.8; p < 0.01) from the anticipated 1:1 female to male ratio (Table 1).
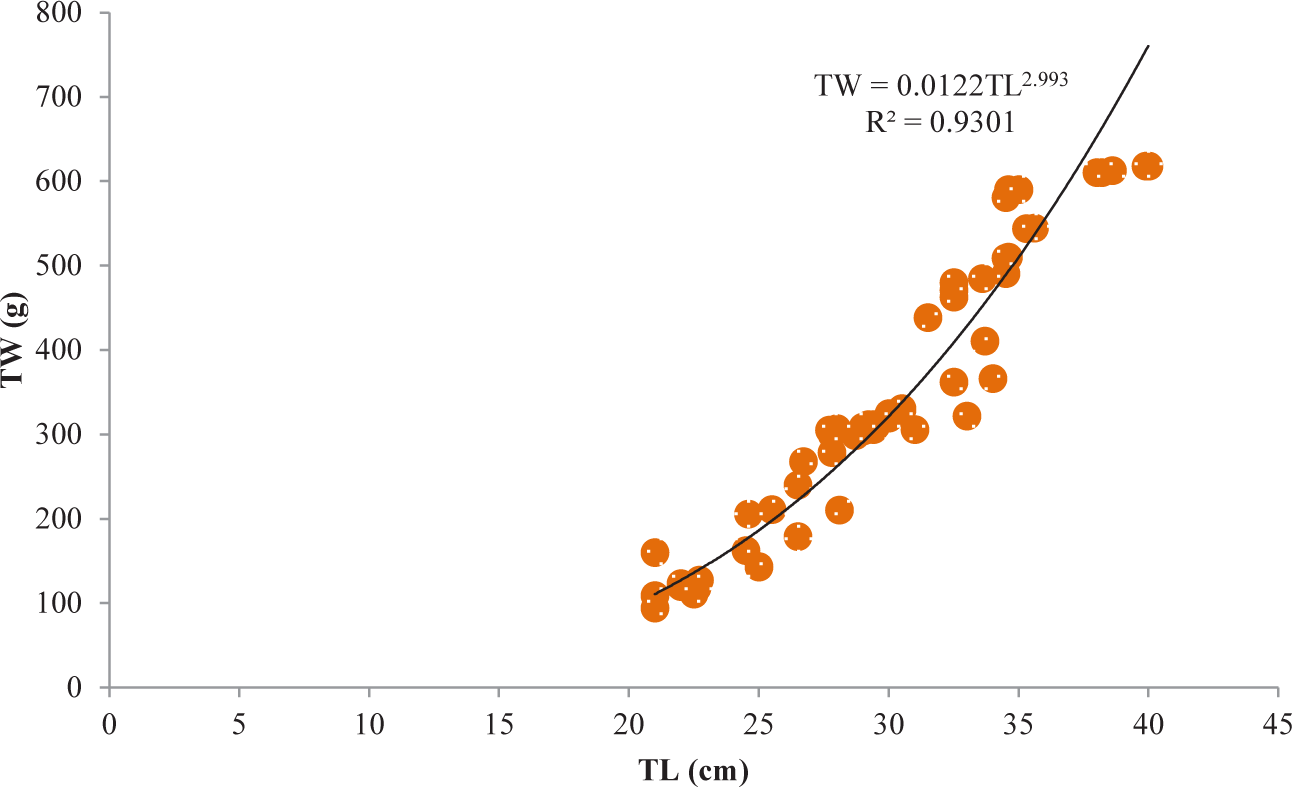
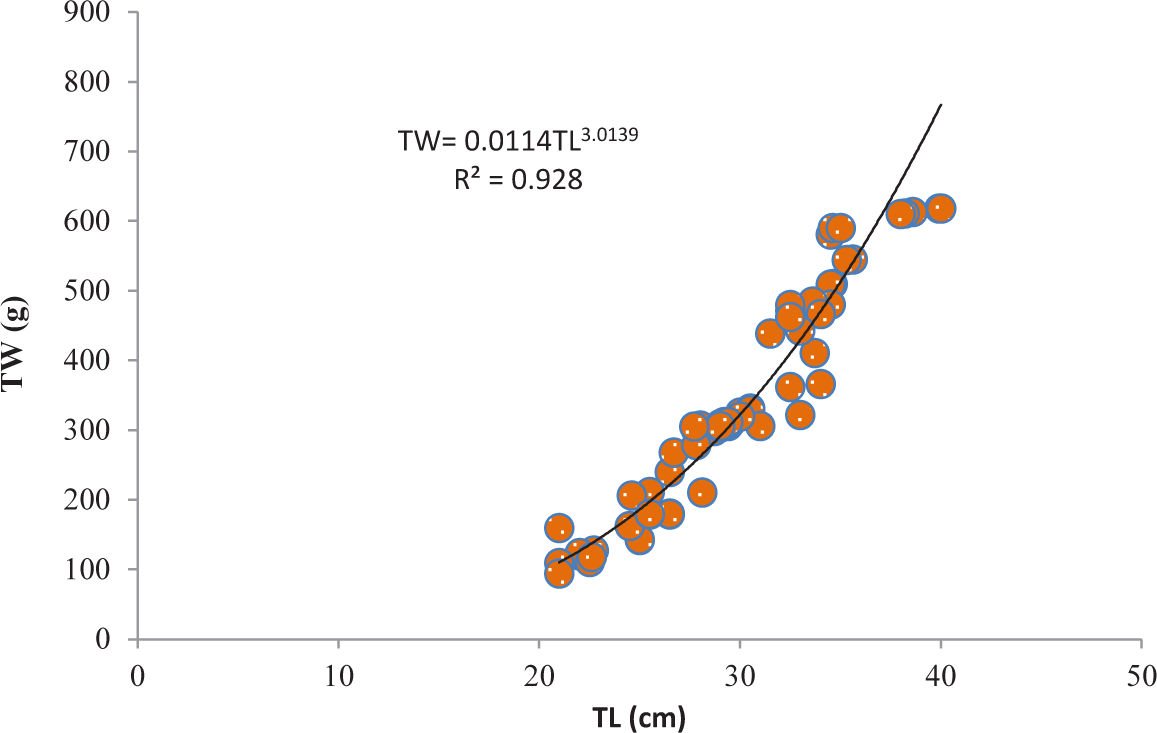
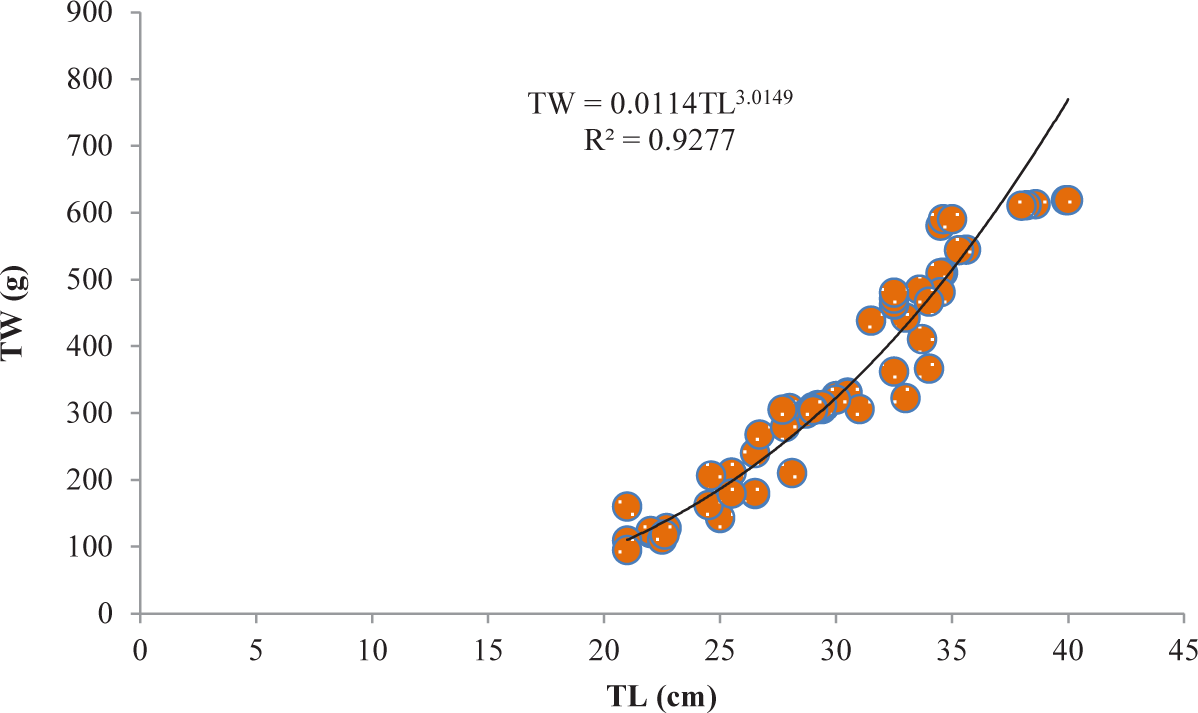
It was discovered that the relationships between TL and TW for V. beso were strongly curvilinear, with a regression equation of TW = 0.0144TL3.0149. The curve-shaped LWRs of the male and female V. beso in the Geray reservoir (TW = 0.0122TL2.993 and TW = 0.0114TL3.0139, respectively) revealed the regression equation’s projected line to be the best fit for the data (Table 2). Both the male (0.9301; Fig 2) and female (0.928; Fig 3) V. beso exhibited r2 values that were close to 1, indicating a strong link between the LWRs of the two sexes. LWRs demonstrated that the slope (b) for V. beso was 3.0149, which was statistically considerably closer to the isometric growth value of fish (b = 3) (p < 0.01) (Fig. 4).
| Fish species | Sex | N | Regression equation | R2 | Sig. |
|---|---|---|---|---|---|
| V. beso | Male | 68 | TW = 0.0122TL2.993 | 0.9301 | ** |
| Female | 102 | TW = 0.0114TL3.0139 | 0.928 | ** |
112 mature fish species (TL: 21.8–40.2 cm; TW: 312–618 g) from populations of V. beso were taken into account for the fecundity estimation. V. beso had absolute fecundity (AF) that varied from 2,190 to 11,265 eggs. AF was strongly correlated with TL, TW, and GW (ANOVA; p < 0.05). However, AF exhibited a greater correlation with GW than TL or TW. Given that the relationship between fecundity and TW had a higher R-value than the relationship between fecundity and TL; it suggests that TW was a stronger predictor of fecundity in this study than TL. There is also a good correlation between length and weight.
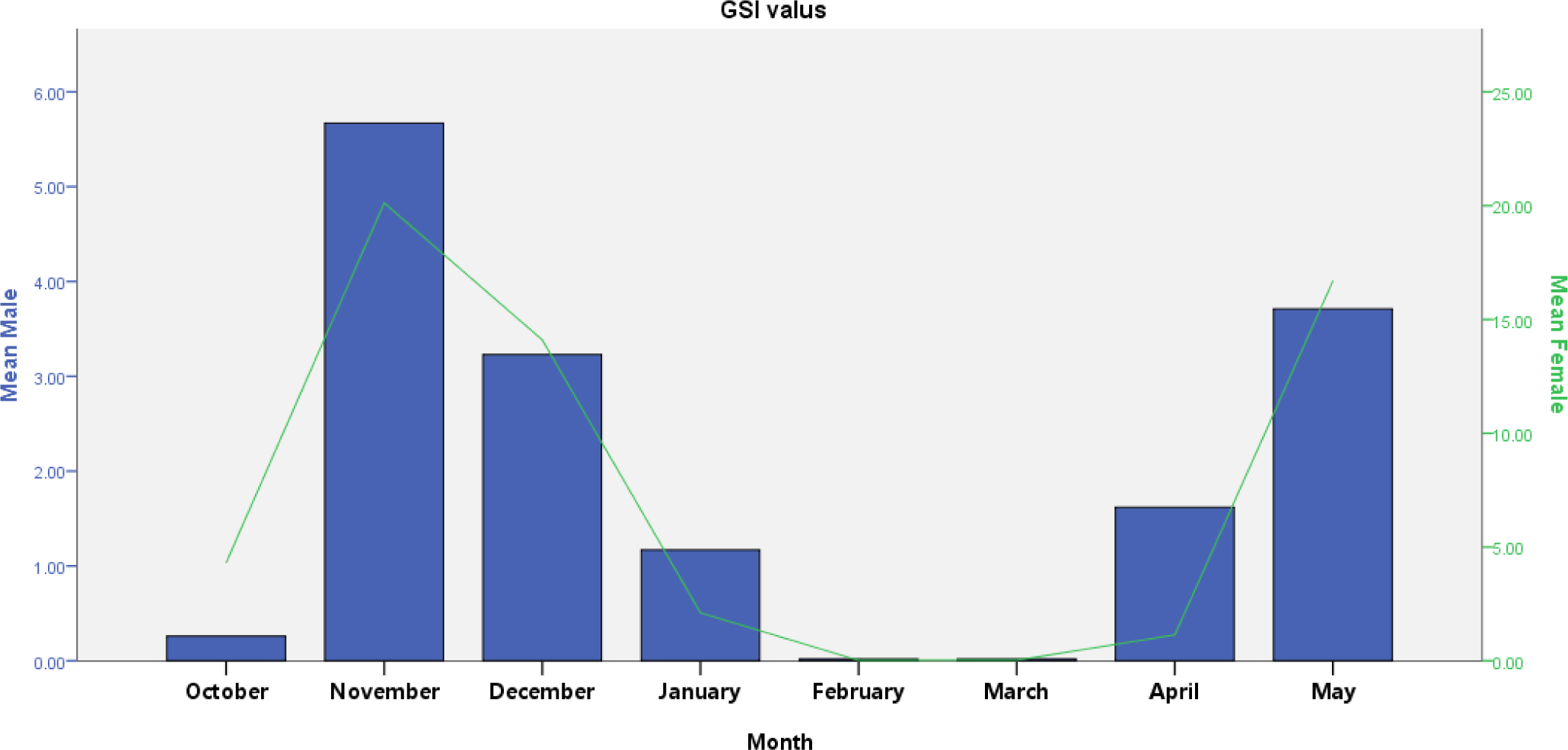
V. beso had a GSI that varied from 0.03 to 5.67 (male) and 0.02 to 20.12 (female). GSI revealed monthly significant variation in both males and females (t-test; p < 0.05). Males had high GSI in November and December, while females had high GSI in November, December, and May (Fig. 5). Peak GSI of male (5.67) and female (20.12) V. beso was seen in November. V. beso had lower GSI values, which were noted in February and March for both sexes (Fig. 5). Monthly GSI estimations showed that the mature captive V. beso population becomes ready to spawn twice, from November to December and April to May, with the peak spawning periods occurring in December. Except for February and March, considerable V. beso population (nearly 90%) was bred from May through December (Fig. 5).
The FCF of V. beso did not significantly vary between sexes (p > 0.05). However, the mean FCF of females (1.48 ± 0.13) was slightly higher than males (1.23 ± 0.09) (Table 3). The FCF of both males and females V. beso varied significantly among the sample months (t-test; p < 0.001). FCF values are typically poor during the spawning period (November, December, April, and May). The mean FCF values of V. beso in this study for both sexes are greater than 1, suggesting the studied species is in a condition of good health.
| Male | Female | |
|---|---|---|
| October | 1.12 | 1.6 |
| November | 1.11 | 1.6 |
| December | 1.3 | 1.1 |
| January | 1.8 | 1.3 |
| February | 1.2 | 2.1 |
| March | 1.1 | 1.8 |
| April | 1.1 | 1.2 |
| May | 1.1 | 1.1 |
| Average | 1.23 ± 0.09 | 1.48 ± 0.13 |
Discussion
The study claims that because of excessive exploitation and inefficient fisheries management (Anteneh et al., 2023b), V. beso in the Geray reservoir is declining. A sex bias exists in the study reservoir, where female V. beso significantly outnumber males. Sexual segregation during spawning, behavioral distinctions between the sexes, and fishing location could all contribute to this sex bias in the study reservoir. The sex gap may be affected by a variety of factors, such as migration patterns, gender-based mortality disparities, and other behaviours (Anteneh et al., 2023a; Engdaw, 2023; Wagaw et al., 2022).
In most of the samples used in this study, males are larger than females. The start of sexual maturity is one factor that might be to blame for size disparities (Admassu et al., 2015). Because they spend more energy on reproduction than males do, females grow more slowly than males do. Because they produce sex reversal and make males grow larger, high water temperatures may also be linked to larger males (Peterson et al., 2005). It’s is also possibly because of intraspecific segregation between the two sexes.
The coefficient (b) values of V. beso in the study reservoir fell within the range of b values (2–4) for fishes in general and tropical fishes in particular (b = 2.5 – 3.5) (Gayanilo & Pauly, 1997). Many researchers have presented thorough isometric and allometric descriptions of the growth patterns of V. beso from various sources of water. The slope of the regression line for V. beso showed an isometric growth pattern, where the fish’s shape and specific gravity do not change as it increases in size. V. beso’s isometric growth in the Geray reservoir demonstrated that it was growing adequately and that this was likely due to the fish’s ability to tolerate pollution or suitable environmental circumstances (Engdaw, 2023). Nevertheless, further research is required to fully understand this theory explaining why fish can tolerate pollution if they are growing isometrically.
It is important to understand fish fecundity to assess fish stock potential and existing fisheries management (Flowra et al., 2012). In the Geray reservoir, the fertility rate of V. beso was comparable to that of Barbus species discovered by Omer (2010) at the head of the Ethiopian Blue Nile. In comparison to other cyprinids such as Barbus grypus (16,000–235,784 eggs), Labeo senegalensis (12,948–74,832 eggs), and Labeo parvus (8,723–124,363 eggs), lower fecundity was discovered in this study (Weil et al., 2013). This variance might be caused by variations in genetic diversity and nutritional accessibility (Teshome et al., 2015). Fish species with these interactions grow quickly (Weil et al., 2013). For the advancement of aquaculture, such a quality is crucial. Offem & Ayotunde (2008) contend that unstable conditions are characterized by fertility, quick development, and early maturation. For V. beso, this was conceivably apparent in the Geray reservoir.
The biology of fish spawning is studied using a coefficient called the GSI (Tagarao et al., 2020). The GSI values found in this study indicated that female had greater GSI values than male. This was due to the ovaries weighing more when eggs were present. In contrast to males, it was discovered that GSI was strongly correlated with the overall length, weight, and stage of gonad maturation of females. This may be caused by the size difference between male and female gonads. The GSI showed that both males and females have monthly fluctuation. The availability of food and the habitat’s year-round warmth may be to blame for the fish’s seasonality in the reproductive period. This study established that the adult captive V. beso population in the Geray reservoir is capable of spawning twice, in November through December and April through May, with December having the highest spawning rates. The ideal period for the reproduction of Barbus sp. in Ethiopia is dependent on external environmental conditions such as rainfall, pH, and turbidity (Mohammed Yesuf et al., 2023).
One of the most popular morphometric indices is Fulton’s condition factor, sometimes known as the “K-factor” and calculated as the ratio of body mass to the cube of length (Batubara et al., 2019). Comparing individual fish of the same length that are either male or female, but only if the female is gravid (carrying eggs), reveals that females typically have a higher k-factor than males do. This is a result of female gonads being larger than male gonads because they contain more eggs. During the spawning season (November, December, April, and May), K-factor values are frequently low. This might be the result of mouthbrooding during the breeding season, which results in famine and a lack of food. Fish frequently reduce their eating activity and use up their lipid reserves while spawning, which lowers the K-factor (Lizama & Ambrosio, 2002). The condition factor is an indicator of the spawning period, since during spawning this factor is low, the fish uses these muscle reserves during spawning period. In this study, the mean k-factor values of V. beso for both sexes are larger than 1, and these values imply that the studied species is in good health. This might be brought on by the availability of food, a suitable habitat, and other environmental factors.
Conclusion
Due to intense anthropogenic pressures and invasions by invasive alien species, the structure of the fish community in the Geary reservoir has lately undergone substantial changes. The African scrape feeder V. beso in the Geray reservoir biological characteristics was originally described in this study. Future fisheries management for fish caught from the Geray reservoir may benefit from the data supplied.