Introduction
Captive breeding programs with the purpose of reintroduction to increase wild populations can be an effective conservation intervention for both game fish fisheries and endangered species (Andrews & Kaufman, 1994; Beck et al., 1994). Captive breeding programs are crucial when wild populations are too limited to support a translocation event (Rakes et al., 1999). There has been substantially less research regarding laboratory culture of endangered and threatened fishes compared to those used for food and recreation (Jason Kline & Bonar, 2011). Reintroduction of captive bred endangered fish species supports the maintenance of ecosystem services and watershed rehabilitation, therefore, is key to whole system restoration. Equally, captive breeding of threatened species can minimize pressure on wild stock and meet the ornamental industry’s growing demand in a sustainable manner by standardizing the method for breeding and fry production.
B. rubra is an Indonesian endemic species belonging to Anabantiformes, Osphronemidae, Macropodusinae; currently known from peat swamps in the Singkil area of Aceh, possibly the Lake Toba area near Medan in North Sumatra, and Sibolga in the northern area of West Sumatra (Hui, 2013). B. rubra is a high economically ornamental fish that plays an important trophic role in the freshwater ecology and very valuable germplasm due to of its endemicity in Sumatra Island (Nur et al., 2020). B. rubra populations have declined due to habitat degradation and loss, over-exploitation of natural populations, introduction of invasive fish species, and global warming (Nur et al., 2020). B. rubra is categorized as endangered in the International Union for Conservation of Nature (IUCN) Redlist database (Low, 2019).
Domesticated fighting fish are primarily derived from Betta splendens (Jason Kline & Bonar, 2011) which have historically been captive-bred as part of cultural heritage and the ornamental trade; however, little is known on the captive breeding of B. rubra. The aims of this study are to provide comprehensive information on the B. rubra captive breed.
Materials and Methods
The study was conducted in compliance with Law of the Republic of Indonesia No. 18 of 2002 on the National System of Research, Development, and Application of Science and Technology. The study was carried out with the Research Institute for Ornamental Fish’s approval and in accordance with ethical principles (Letter of Assignment from head of the research institute 56/BRSDM-BRBIH/HK.60/I/2018).
The study was conducted at the betta fish hatchery at the Research Institute for Ornamental Fish, Depok, Indonesia. Juvenile fish were captured from rivers near peat swamps in Aceh, Indonesia by local fishers to be used as brood stock (Figs. 1 and 2A). A total of 100 pairs of fish (approx. 5 cm total length) were bagged individually using polyethylene (PE) plastic sized 10 × 25 cm with ratio of water and air 1 : 3, then placed into a styrofoam box and shipped by delivery for three days (Fig. 2B).

Fish were acclimated by floating each plastic bag in the rearing medium to slowly adjust the water temperature. Females were kept together in a betta sorority in a holding tank (40 × 40 × 60 cm3 or 96 L in volume) and males were reared in solitary conditions in smaller holding tanks (10 × 10 × 25 cm3 or 2.5 L in volume). Holding tank water was conditioned to match natural soft blackwater habitat using catappa leaves. The fish were fed with bloodworm (Chironomus sp. larvae) and/or mosquito larvae (Culex sp.) three times per day to satiation. Fish rearing was carried out for approximately 45 days until the fish were mature and ready to be spawned.
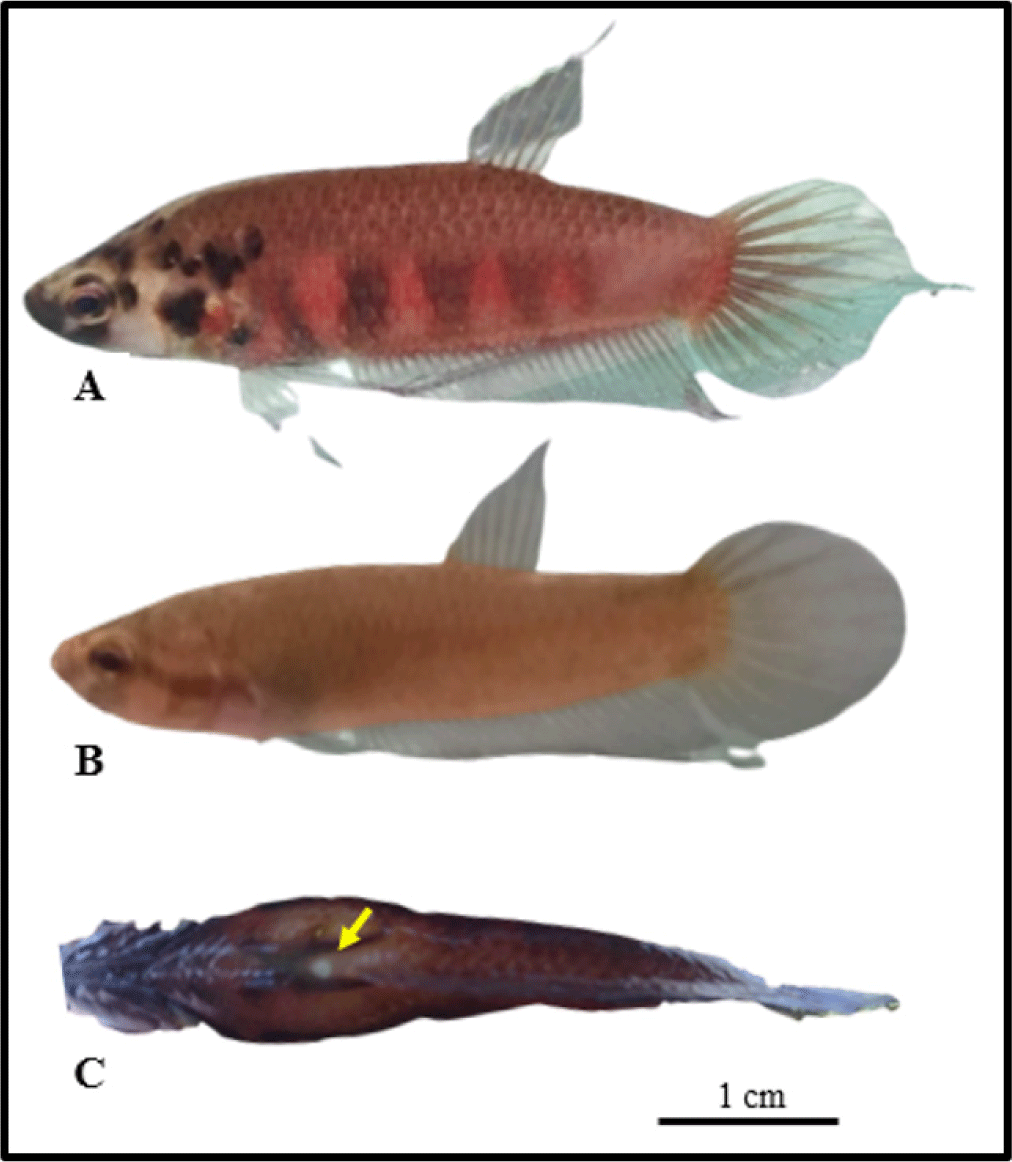
Before breeding, the broodstock were selected based on their healthy physical characteristics, absence of any physical impairments, and maturity characteristics. Mature males were distinguished from a more vibrant body color, while mature females were characterized by a bulging stomach and white dot on the genital pore (Fig. 3).
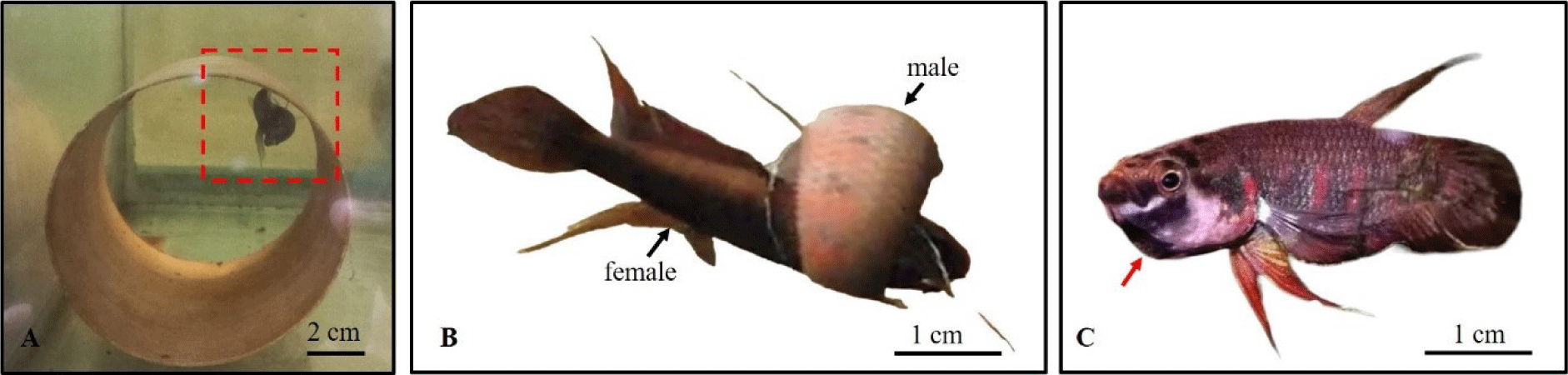
Selected males and females were put together in pairs in cylindrical breeding tank (height 30 cm, diameter of 35 cm), with dried catappa leaves to maintain optimal soft blackwater conditions, for spawning. A polyvinyl chloride (PVC) pipe (length 10 cm, diameter of 10 cm) was placed inside the plastic jar as a shelter. Spawning occurred after 1–2 weeks. During the spawning event the male and female entangle around each other (Fig. 4A and 4B) and the female releases 3–5 eggs. The male then picks up the eggs and mouthbroods. This process is repeated 10–15 times until all the eggs are released. After spawning finished, the female was taken from the spawning vessel and the male was left to incubate the eggs in his mouth (Fig. 4C) until they hatched.
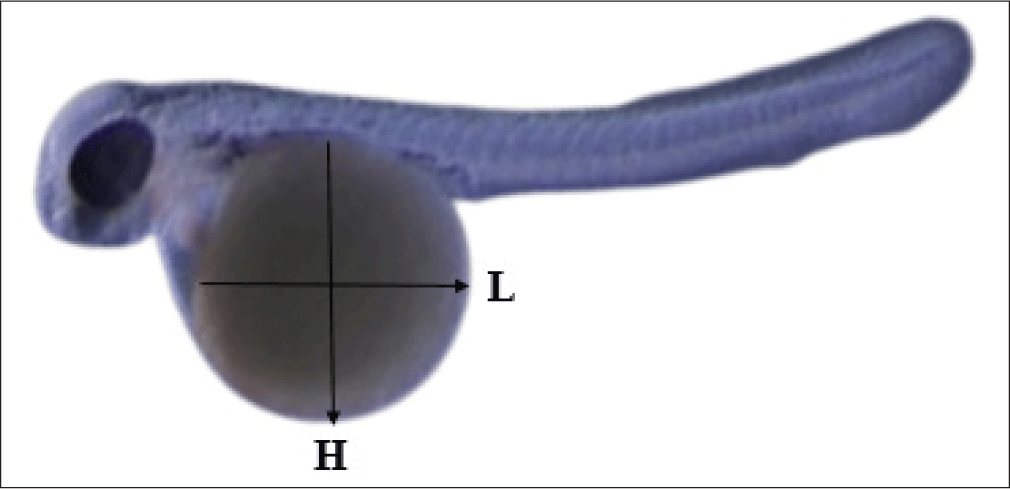
The eggs were taken from different mouthbrooding male after 1 days post spawning (dps), 2, 3, and 4 dps (eggs were not developed after being taken in 1–3 dps). The observation was continued using eggs collected at 4 dps and incubated outside of the mouths of the male parents until the yolk was fully absorbed in 12 dps. Under a light microscope linked to a video monitor, embryogenesis (1–5 dps) and yolk absorption (6–12 dps) were observed. The volume of the yolk was measured to determine its absorption rate (μm3/hours). The yolk volume (Vy) and absorption rate (YAR) were determined using the formula:
where L and H are the length (μm) and height (μm) of the ellipsoidal mass of the yolk (Fig. 5) (Heming & Buddington, 1988). V0 and Vt are the initial and last measured yolk volumes (μm3); t is the time difference between the first and final measurements (hours).
The 4 dps-old eggs were externally incubated until 6 dps in order to observe the hatchability of eggs outside the male mouth. The hatching rate (HR) was then calculated by counting the number of hatched larvae. The formula for calculating the HR as a percentage was as follows:
where Ne represents the number of eggs, while Nl represents the number of hatched larvae.
A total of 24 newly-hatched larvae were transferred equally into three aquarium with size of 15 × 15 × 20 cm3 filled with two liters of water.
Three different live feeds were assessed to determine ideal feed for initial first eight days of larval rearing, these were Artemia, Moina, and Tubifex. Larvae were obtained from males that had been mouthbreeders for 12 dps. A total 45 larvae were reared in nine glass aquaria (15 × 15 × 20 cm3). Each treatment were consisted of three replication. In rearing, larvae were feed at satiation three times per days following treatments. In Tubifex feeding treatment, the worms were chopped to adjust the size of the larva’s mouth opening. Tubifex worm was chopped with size of 114–229.7 µm so that B. rubra larvae are able to consume it as their gape is around 385.2 µm. The parameters were observed in this experiment are fish length growth and survival rate (SR). SR were calculated based on the formula:
where Nt is final total larvae and Nh is total initial larvae.
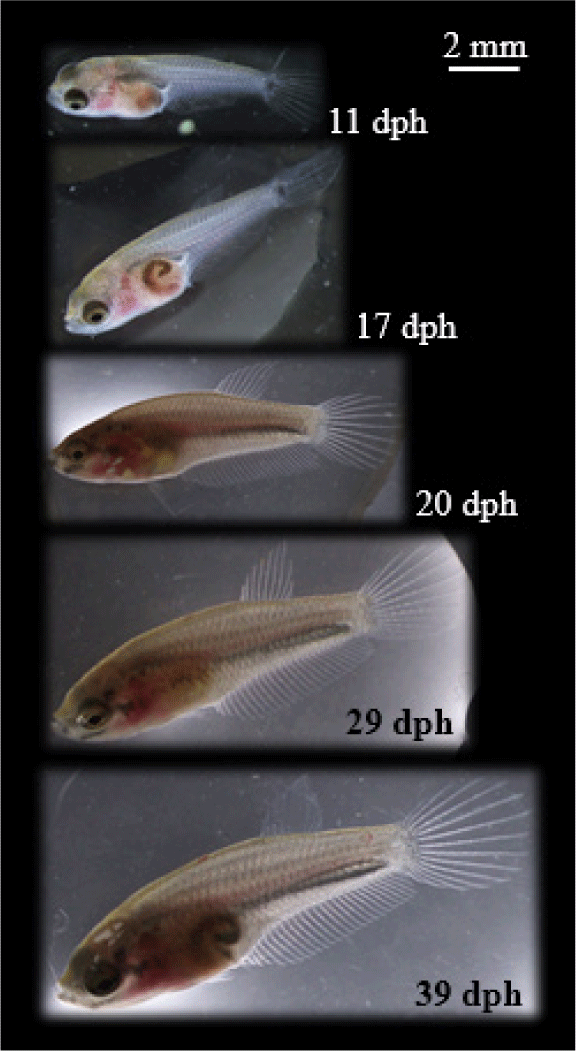
After the optimal feed type was determined in the first feeding experiment, mass rearing of larvae using the optimal feed (Tubifex) was carried out from day 1 after hatching until days 39 when larvae become definitive fry with ad satiation feeding. In total 200 larvae were reared in a glass aquaria (100 × 40 × 25 cm3) with 15 cm height of media. We added five pieces of dried catappa leaves into the media to create brown water conditions to emulate natural conditions. Length of larvae were measured in days 1, 7, 17, 29, and 39 to observe the growth length. SR was observed using same formula in feeding experiment.
Growth and reproductive performances across two generations (G1 and G2) were assessed using a total 150 fry (40 dah), where each generation was reared equally in three styrofoam box (40 × 40 × 60 cm3) until they reach the adult stage for 18 weeks or 6 months. Weight and length were measured every three weeks for observed the growth. After 6 months, male and female were reared separately. Males were reared on solitary glass aquaria (15 × 15 × 20 cm3) and female were mass reared in styrofoam box (40 × 40 × 60 cm3). Bloodworm (Chironomus sp. larvae) and mosquito larvae was fed alternately, ad satiation, two times per days at 07.00 AM and 04.00 PM. Male and females were reared until ready to spawn. Reproductive parameters were observed: fecundity (total egg produced), total hatched larvae, and HR. The eggs were taken from different mouthbreeder male after 5 dps and were externally incubated until 6 dps in order to observe the fecundity of female broodstock and hatchability of eggs outside the male mouth.
Most of the data were analyzed descriptively, only feeding kind treatment data were analyzed statistically analysis of variance (ANOVA) with 95% level of confidence after checking for normality of data with Kolmogorov Smirnov test; post-hoc tests were used to assess differences between levels using the Duncan Multiple Range Test (DMRT) with SPSS (7.0) statistical software. Linear relationship and trend line analysis were conducted using Microsoft Excel.
Results
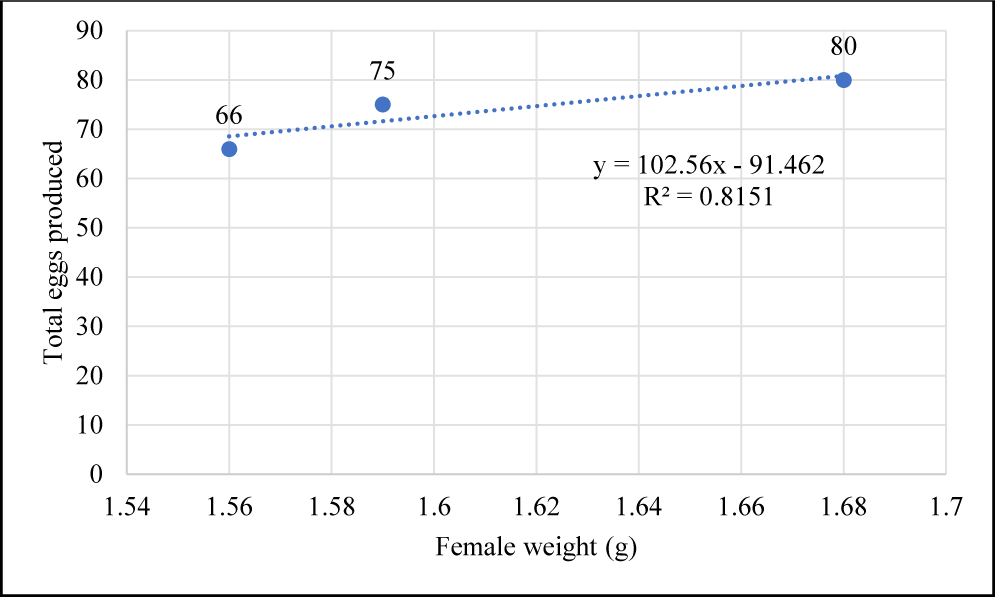
In this study, B. rubra breeding was successfully achieved under laboratory conditions. Table 1. summarizes the outcomes of B. rubra breeding trials under laboratory conditions. We found that the female with total length 5.17 ± 0.15 cm and weight 1.61 ± 0.06 g produce, on average, 73.67 ± 7.09 eggs, total larvae 34.33 ± 5.13, and HR 46.67 ± 5.77 %. Female weight is directly proportional to the number of eggs produced (Fig. 6) following a positive linear equation y = 102.56x – 91.462 with R2 = 0.8151.
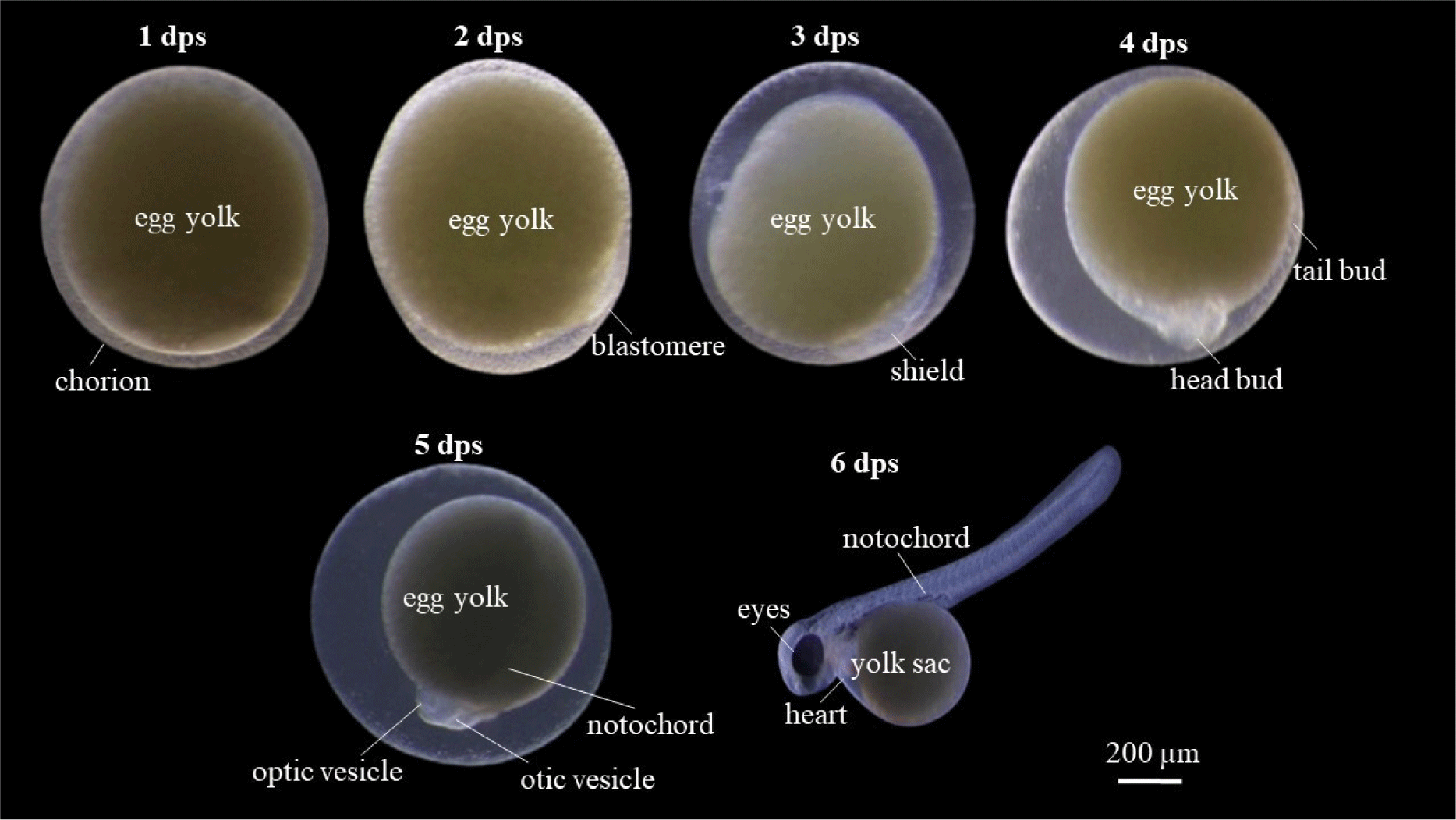
Embryogenesis process occurred for 1 dps until the egg hatched in 6 dps (Fig. 7). B. rubra eggs are about 2 mm in size, at 1 dps was the initial phase of cleavage, a clear chorion was visible (a sign of a fertile egg), the yolk was yellowish, slightly cloudy and not translucent. The blastula phase were occurred at 2 dps and indicated with multiple cell divisions with visible blastomeres. Blastomeres are zygote cells that have divided by mitosis to become 64 cells. When the zygote divides to become 64 cells, the blastomeres are round like grapes, which are also called morula. Gastrula phase was observed at 3 dps, blastomere shows invagination and forms a cavity called gastrocoel, blastomer then covers 50% of the yolk (epiboly) which indicates the ongoing embryo shield (embryo shield) of egg yolk. Stage of organogenesis was obtained at 4 dps, the head bud at the animal pole and tail bud at the vegetal pole has been seen, and the epiboly has been closed 100%. Advanced organ formation phase was occurred at 5 dps, optic vesicles or eye sockets have been formed which is followed by the formation of the notochord or spinal axis. The otic vesicle was already visible in this embryonic stage and was behind the head which is shaped like a bubble with two dots. The eggs were hatched at 6 dps.
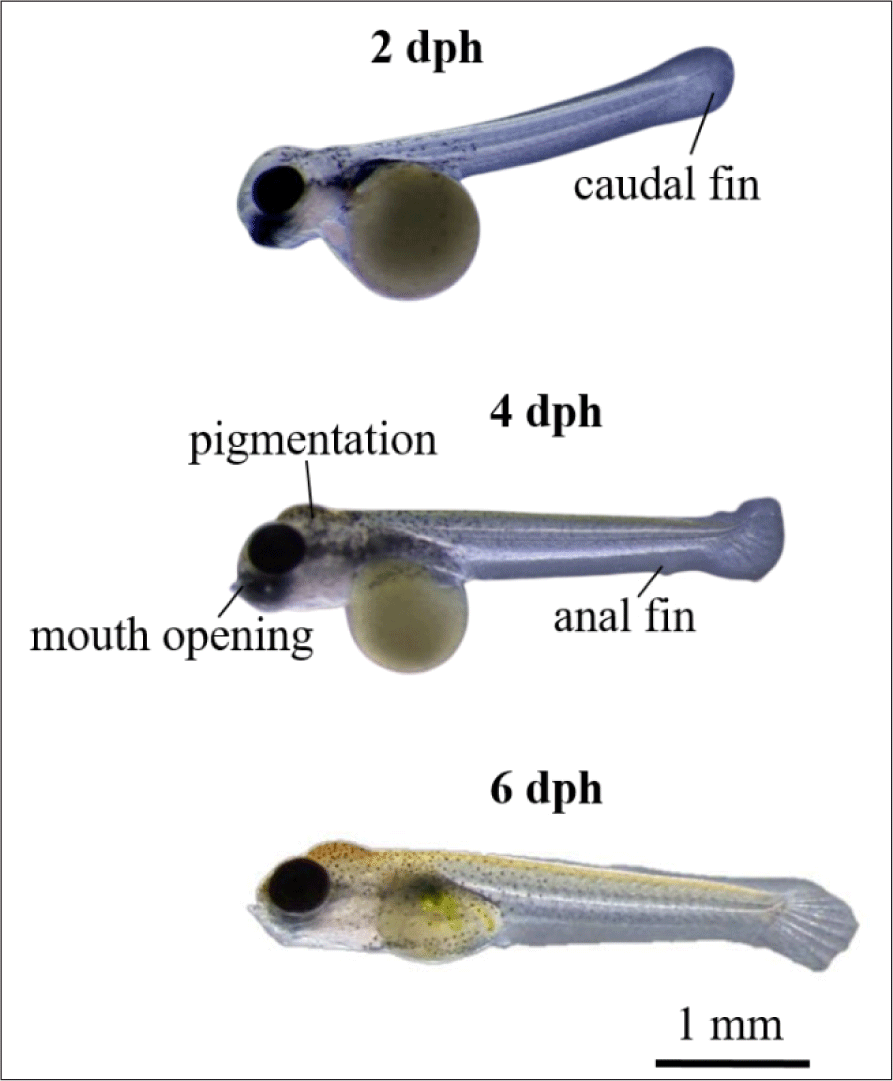
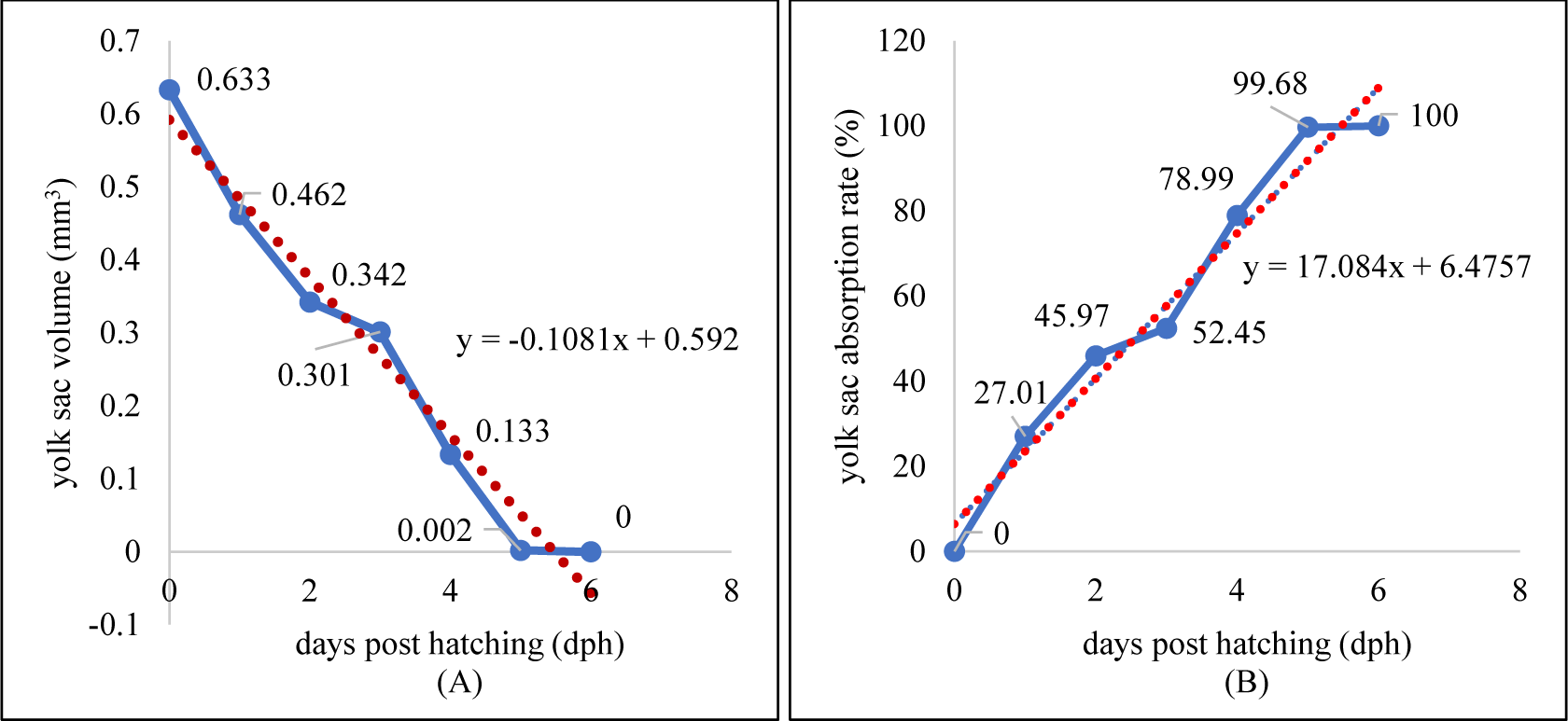
Larvae development and yolk absorption were obtained at 0–6 dph (days post hatching). Pigmentation was starts to appear at 2 dph and looks more at 4 dph (Fig. 8). The newly-hatched larvae had yolk with volume of 0.633 mm3 as endogenous feed source. The volume of yolk decreasing along as the larvae developed and get older. The volume of yolk was 0.462 mm3 at 1 dph, 0.342 mm3 at 2 dph, 0.301 mm3 dph, 0.133 mm3 dph, 0.002 mm3 dph, and the yolk fully absorbed at 6 dph (Fig. 9A). The decreasing volume of yolk of larvae was related to yolk absorption used in larval organ development. The percentage of yolk absorption rate of larvae indicated a percentage of 27.01% at 1 dph, 45.97% at 2 dph, 52.45% at 3 dph, 78.99% at 4 dph, 99.68% at 5 dph, and 100% completely absorbed 6 dph (Fig. 9B). The volume of yolk decreasing indicates negative linear equation y = –0.1081x + 0.592, the yolk absorption rate follows positive linear equation y = 17.084x + 6.4771.
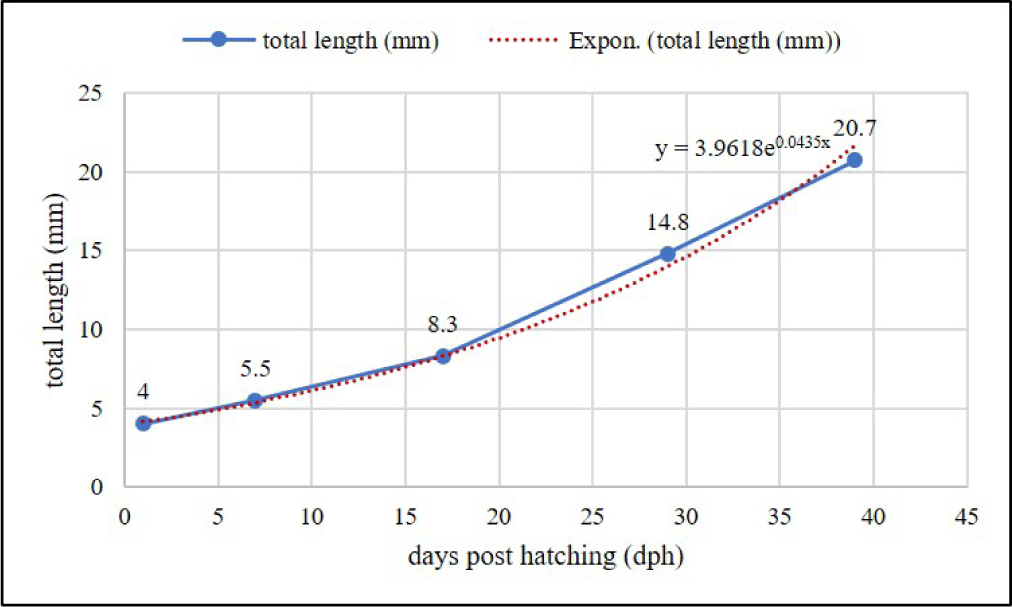
The larvae first feeding of chopped Tubifex has effects on the greatest increase of length, followed by feeding Artemia and then Moina (Table 2). Meanwhile, the difference in feed did not have a significant effect (p > 0.05) on larvae SR. B. rubra larvae were grown and developed normally into fry in the captivity medium indicated by slow growth at the beginning of rearing (1–7 days) and increasing rapidly (exponential phase) at the age of 8–39 days. Growth pattern of larvae following exponential equation y = 3.9618e0.0435x (Fig. 10). Development of B. rubra larvae into fry can see in Fig. 11.
| Parameter | Treatment | ||
|---|---|---|---|
| Artemia | Moina | Tubifex | |
| Increase of length (mm) | 2.438 ± 0.542ab | 1.206 ± 0.723a | 2.677 ± 1.125bc |
| Survival rate (%) | 100 ± 0.000a | 93 ± 11.547a | 100 ± 0.000a |
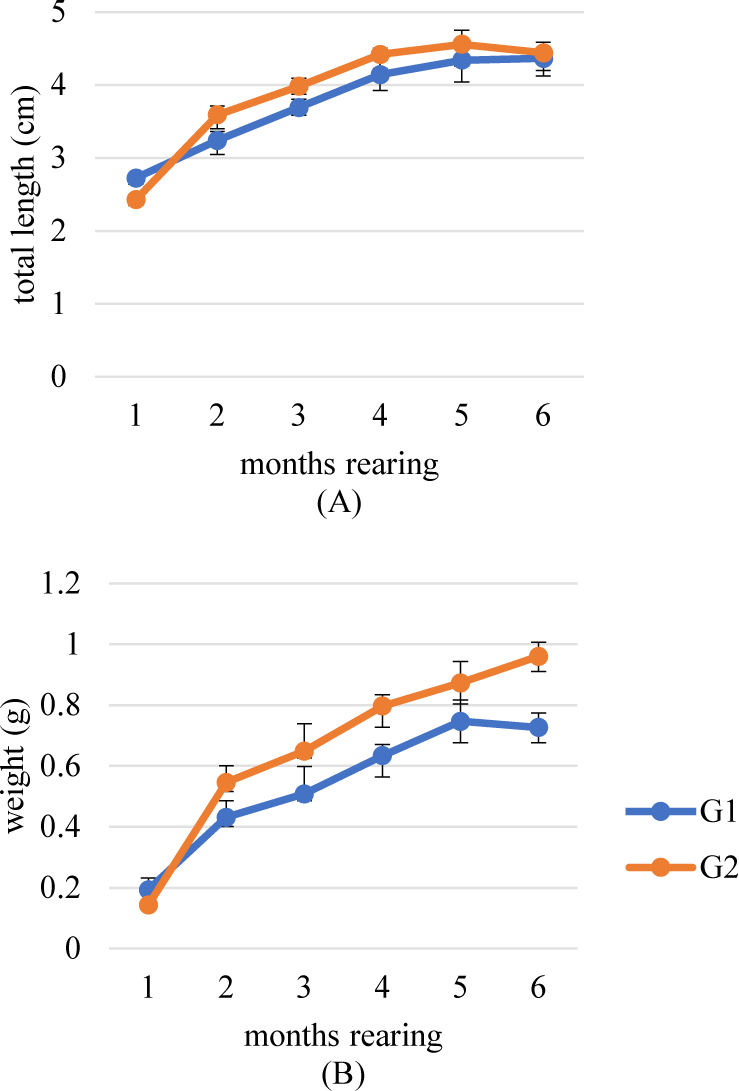
We succeeded in breeding B. rubra to produce 2 generations on captivity media which was shown in its growth and reproductive performance in each generation. The first and second generations (G1 and G2) were shown a similar growth pattern, but G2 tends to grow in length and weight which is better than G1 (Fig. 12). Meanwhile, the reproductive performance did not show a significant difference (p > 0.05) between the broodstock of wild origin (G0) with G1 and G2 (Table 3).
| Generation | Fecundity | Total hatched larvae | Hatching rate (%) |
|---|---|---|---|
| G0 | 72.00 ± 6.24 | 38.00 ± 4.58 | 52.68 ± 1.85 |
| G1 | 72.67 ± 11.01 | 39.00 ± 8.72 | 53.24 ± 4.28 |
| G2 | 74.00 ± 6.93 | 40.00 ± 5.00 | 54.01 ± 3.65 |
Discussion
B. rubra has been bred by several hobbyist breeders but has never been reported in an international scientific manuscript. In this study we report our success in B. rubra breeding under laboratory conditions across two generations in captivity. We conducted several experiments and observations to obtain data from several parameters that can be used as a reference in further ex-situ conservation and domestication of B. rubra.
According to the research, the quantity of eggs produced is directly correlated with the weight of the female. The equation y = 102.56x – 91.462 with R2 = 0.8151 shows a positive linear connection, indicating that larger females often lay more eggs. Egg size and amount are positively correlated with fish size in several fish species. In Salvelinus fontinalis and Salvelinus alpinus, both absolute fecundity and egg volume increase with body size (Purtscher & Humpesch, 2006). Similarly, in five species of fork-tailed catfish, mature egg weight is positively correlated with female parent length (Coates, 1988). The diameter and dry weight of haddock eggs are also positively correlated with fish length, with smaller, younger fish having smaller and lighter eggs (Semmens & Swearer, 2012). In Tilapia zillii, fecundity increases with maternal size, and there are significant relationships between fish size and various indices of egg size (Hislop, 1988).
There is a data deficit on mouthbrooding betta fecundity, and reproductive ecology in general. Nonetheless, the number of B. rubra eggs is far less than the number of eggs that can be produced by bubblenest betta such as B. splendens which can produce up to more than 400 eggs (James & Sampath, 2003). Eggs were incubated outside the male’s mouth to be able to observe the process and the HR externally. If the eggs are allowed to hatch in the males’ mouth, around 60–80 larvae can be produced – i.e. more than the number of larvae which can hatch outside the male’s mouth. Eco-evolutionary processes ensue that the environmental conditions in the male mouthbrooders mouth is optimal for egg development. Egg incubation can produce the same HR if completed in an artificial incubator that mimics the conditions in the male’s mouth, such as proper aeration and stirring of the eggs (James & Sampath, 2003).
Understanding B. rubra’s early life stages and reproductive biology is crucial for managing captive breeding and ensuring the survival of the species. The development of lotic-breeding species in captivity has been studied, and it has been found that genetic and environmental factors influence different aspects of larval development (Tanaka & Nishikawa, 2022). In the case of the Pyrenean sculpin, a successful captive breeding program was implemented, and different phases such as nesting behavior, courtship, egg fixation, parental care, hatching, and survival during juvenile development were identified as critical for success (Manubens et al., 2020).
The research provides a thorough analysis of yolk absorption in B. rubra larvae. The data demonstrate a steady reduction in yolk volume as the larvae grow, with full absorption occurring six days after hatching. The significance of tracking yolk absorption throughout the early stages of larval development is emphasized by the positive linear equation for yolk absorption rate (y = 17.084x + 6.4771). The absorption rate of fish egg yolk varies depending on various factors such as size of the eggs and larvae, the volume of the yolk, the incubation temperature, oxygen concentration, and metabolic activity of the absorptive layer (Jardine & Litvak, 2003; Mero et al., 2022; Pepin et al., 1997; Reyes-Syafariyah et al., 2023). Yolk absorption occurs in a sequence, with yolk platelets being mobilized more rapidly than the oil globule after hatching (Kamler, 2008). In the case of Dormitator latifrons larvae, the highest percentage of yolk absorption (52%) occurs within 24 hours post-hatching, and the yolk sac is completely reabsorbed at 96 hours post-hatching (Heming & Buddington, 1988). The rate of egg yolk absorption offers critical information about when it is appropriate to feed the larvae for the first time. After six days of yolk absorption, B. rubra larvae was feed for the first time with some kind of feed.
The type of initial feeding has a significant impact (p < 0.05) on the growth of B. rubra larvae. Chopped Tubifex is the most effective in promoting growth, followed by Artemia and Moina. Initial feeding in fish larvae is a critical period that significantly (p < 0.05) impacts their survival and growth (Lahnsteiner et al., 2023). Prior studies conducted on the nourishment of goldfish larvae have yielded consistent findings, indicating that Tubifex manifests superior outcomes with respect to both SR and growth in comparison to moina and artemia (Mellisa et al., 2018). Tubifex has a higher protein content compared to other live feeds, has a bright color and special smell that attracts fish, easily consumed by larvae due to its feeding habits, and has a lower movement activity, making it easier for fish to capture (Conceição et al., 2010; Mellisa et al., 2018). The lowest growth and SR were found in Moina feeding treatment. The lack of nutrients in Moina can have significant effects on its growth and survival. The feeding regime for initial feeding is species-specific, and optimal regimes should be established for each new species (Sommerfeld & Holzman, 2019). The study findings have practical implications for optimizing the diet of B. rubra larvae in captivity to support their growth and development.
The growth pattern of B. rubra larvae is characterized by slow initial growth during the first 7 days and rapid exponential growth from day 8 to day 39. This information is valuable for managing the rearing of B. rubra larvae, allowing for appropriate care and nutritional support during different growth phases. The growth-survival paradigm for fish larvae is supported by the positive association between feeding success and growth across all taxa. The possibility for an early “critical period” regulating survival differs among species, reaching a maximum in fast-growing fish, according to both the correlation between feeding success and growth and the serial correlation of growth time-series (Pepin et al., 2015). Growth pattern of B. rubra larvae following exponential equation y = 3.9618e0.0435x. Fish larvae exhibit exponential growth patterns (Manabe et al., 2018). The feeding activity of fish larvae is described by an equation that includes parameters for the area successfully searched, larval swimming speed, and the time required to consume food energy (Brahman & Chandra, 2016). The proportion of ingested food energy used for metabolism increases exponentially with increasing swimming speed (Renner-Martin et al., 2018).
The research successfully bred two generations (G1 and G2) of B. rubra in captivity. G2 showed better growth in terms of length and weight compared to G1, indicating the potential for improvement through selective breeding. However, it is noteworthy that reproductive performance did not significantly differ (p > 0.05) between the captive-bred generations (G1 and G2) and the wild-origin broodstock (G0). Fish captive breeding that leads to domestication shows changes from generation to generation in various aspects such as behavior, growth, physiology and metabolism, phenotypic diversity, and reproduction (Liao & Huang, 2000). Atlantic salmon (Salmo salar) provided evidence that domestication can increase growth rate (Fleming et al., 2002). Domestication has an impact on the age of sexual maturation. Milkfish (Chanos chanos) mature in various locations under various circumstances. Compared to the six to seven years maturation for fry taken in the wild, an earlier maturity of five to six years was attained for fish generated in hatcheries (Liao, 1991). The impact of captive B. rubra on growth and reproduction needs to be thoroughly studied further.
The findings contribute to the understanding of how domestication may impact B. rubra’s growth and reproduction. While there are hints of improved growth in captivity, further research is needed to comprehensively assess the long-term effects of domestication, including potential changes in behavior, physiology, and phenotypic diversity. These insights are crucial for the conservation and sustainable management of this species.
Conclusion
In conclusion, our study highlights significant findings regarding the captive breeding of B. rubra. The study findings indicate that female B. rubra, with an average length of 5.17 ± 0.15 cm and weight of 1.61 ± 0.06 g, typically produced around 73.67 ± 7.09 eggs, resulting in approximately 34.33 ± 5.13 larvae, with a hatching rate of approximately 46.67 ± 5.77%. Embryogenesis began at spawning and concluded when the eggs hatched, typically occurring within six days. Larvae development and yolk absorption took place during the initial six days post-hatching. Assessing various initial feeding options revealed that chopped Tubifex resulted in the most significant increase in length. The growth pattern of B. rubra larvae displayed slow initial growth in the first seven days, followed by a rapid exponential growth phase until day 39. Successful breeding of two generations (G1 and G2) in captivity was achieved, with G2 exhibiting superior growth tendencies compared to G1. Importantly, reproductive success did not significantly differ between wild-origin broodstock (G0), G1, or G2. These findings contribute valuable insights into conservation and captive breeding strategies of B. rubra, emphasizing the importance of continued research and collaborative efforts to ensure the long-term survival of this endangered species.
