Introduction
Functional foods containing biological and physiological active compounds are emerged as dietary supplements for preventing various diseases such as hypertension, diabetes, cardiovascular disease, obesity, etc (Bigliardi & Galati, 2013; Mondal et al., 2021). In response to the rising awareness of health benefits, demand for functional foods have increased significantly (Bigliardi & Galati, 2013; Block et al., 2011). In addition, previous study has reported that the functional labeling foods market expects to revitalize the food industry and promote the development of functional materials or ingredients (Choi, 2022).
The interest of functional ingredients for immune health increased during the emergence of COVID-19. Previous studies have shown that several functional ingredients can help support the immune system (Hamulka et al., 2020; Mullin et al., 2021). The immune system plays an important role to maintain the stability of internal environment and prevent diseases against infectious agents (Nourbakhsh et al., 2022). Particularly, the dietary antioxidants prevent the cell damage and improve both the innate and adaptive of the immune system (Pangrazzi, 2019). Over the past few years, Aloe vera has been utilized as a healthcare product in the world. According to the previous studies, polysaccharides isolated from A. vera contribute to bioactive properties including anti-oxidant, immunostimulation, anti-inflammation, and anti-viral (Kang et al., 2014; Liu et al., 2007; Ni et al., 2004). In addition, red ginseng extracts, as health functional ingredients, possess many biological activities such as anti-oxidant, improvement of immunity and blood circulation.
Surimi, also known as a seafood, is a unique functional food ingredient made from refined fish myofibrillar proteins (Xiong et al., 2009). Also, surimi products have reflected by steady increase in global consumption due to their unique texture and nutritional values including the low levels of cholesterol, high-value proteins, and wide range of nutrients (Endoo & Yongsawatdigul, 2014; Tabilo-Munizaga & Barbosa-Cánovas, 2004). Surimi-based products have become common items of processed foods such as eomook, fish balls, fish tofu, fish sausage, kamaboko, bamboo wheel, and imitation crab meat (Park, 2000; Vidal-Giraud & Chateau, 2007). In addition, previous studies have been conducted the effects of gel properties and flavor characteristics from surimi which contain bioactive ingredients (Sharma et al., 2023; Walayat et al., 2022; Zheng et al., 2021).
In the present study, surimi products in including an A. vera gel powder and a Red ginseng extract were prepared to improve immunity. The surimi products were compared with/without the ingredients in in vitro and in vivo models after an artificial digestion.
Materials and Methods
Frozen Alaska Pollock (Theragra chalogramma) surimi (KB grade) was purchased from Jeju Tamna Seafood (Jeju, Korea). A. vera gel powder and Red ginseng extract were provided by Shinwoo (Yeoju, Korea) Dulbecco’s modified Eagle’s medium (DMEM), trypsin, phosphate-buffered saline (PBS), Fetal Bovine Serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT), sulfanilamide, N-(1-Naphthyl)ethylenediamine dihydrochloride, phosphoric acid, 2’7’-dichlorogluorescein diacetate (DCF-DA), acridine orange, and diaminofluorescein-FM diacetate (DAF-FM-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The functional surimi product (FSP) was prepared based on the methods reported by Oh et al. (2019). We made two types depend on with/without functional ingredients, referred to as general surimi product (GSP) and FSP. To prepare FSP, the frozen surimi was thawed and ground at 4°C using a refrigerated food cutter for 20 min. And then, 2% salt, 2% starch, A. vera gel powder and red ginseng extract were added and ground for an additional 20 min. The FSP contains the A. vera/red ginseng extract ratio of 0.4:0.0 to 0.0:0.4 (Table 1). The mixed surimi was stuffed into a polyvinylidene casing with a diameter of 2 cm, and were sealed both ends of the casing. And then, GSP and FSP were prepared by heating in water bath at 95°C for 30 min. The heated FSPs were cooled immediately in ice water for 30 min and stored at 4°C.
| Quantity (%) | GSP | FSP | ||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | ||
| Aloe vera | NA | 0.4 | 0.3 | 0.2 | 0.1 | - |
| Red ginseng extract | NA | - | 0.1 | 0.2 | 0.3 | 0.4 |
Gel strength was evaluated using a Sun Rheo Meter CR-100 (Compac-100, Sun Scientific, Tokyo, Japan) with a diameter of 5 mm cylindrical plunger and compression speed of 20 mm/min. Gel properties was investigated by measuring gel strength (g × cm).
Each artificial digestion solution was prepared based on the pre-described method by Oh et al. (2022). The compositions of the two artificial digestive solutions showed in Table 2. Briefly, 300 g of GSP and FSP were digested by gastric solution (pH 2, 1 L) and incubated at 37°C for 4 h. After adjusted to pH 8, 1 L of duodenal solution were added and incubated at 37°C for an additional 4 h. And then, the samples were inactivated digestive enzymes by heating in water bath at 95°C for 10 min. The digests of GSP and FSP were prepared by collected the supernatant after centrifugation. The digests of GSP and FSP was used for further in vitro and in vivo experiments.
Previous studies have reported that Vero cells represent an in vitro model to study the effect of antioxidant (Oh et al., 2022; Wang et al., 2019). In addition, RAW 264.7 cells as a macrophage were used to research the immune response (Jayawardhana et al., 2023; Yang et al., 2023). Both of Vero cells and RAW 264.7 cells were maintained in DMEM included 10% FBS and antibiotics, at 37°C in a humidified incubator with 5% CO2. Cell viability were determined by the mitochondrial activity to convert MTT solution (2 mg/mL) to an insoluble formazan product. Briefly, Vero cells were treated with 500 µg/mL of GSP and FSP, incubated for 1 h, and subsequently added with 0.3 mM H2O2 for an additional 24 h at 37°C. RAW 264.7 cells were treated with 500 µg/mL of GSP and FSP, incubated for 24 h at 37°C. Each cell was pretreated with MTT solution for 3 h. Each cell density was assessed by measuring optical density (OD) at 540 nm using a microplate reader (BioTek, Winooski, VT, USA).
The intracellular reactive oxygen species (ROS) production were evaluated by DCF-DA assay in accordance with previous methods (Kang et al., 2019). Vero cells were treated with 500 µg/mL of GSP and FSP, incubated for 1 h, and subsequently added with 0.3 mM H2O2 for an additional 24 h at 37°C. After 24 h of incubation, 10 µM DCF-DA solution was added and incubated for 10 min at 37°C in the dark. The fluorescence intensity was detected by microplate reader (BioTek) at an excitation and emission wavelength for 485 nm/535 nm.
Measurement of nitric oxide (NO) production was analyzed the immune response using Griess reagent containing 1% sulphanilamide, 0.1% napthylethylenediamine dihydrochloride, and 2.5% phosphoric acid (Yang et al., 2023). RAW 264.7 cells were treated with 500 µg/mL of GSP and FSP, incubated for 24 h at 37°C. The supernatants were treated with Griess reagent and incubated for 10 min at 37°C. The OD was read at 450 nm using a microplate reader (BioTek).
All experiments were conducted following the experimental animal guidelines of Jeju National University animal center. Ethical approval was obtained from the Animal Care and Use Committee of the Jeju National University, Korea (Approval No. 2022-0005).
Maintenance of zebrafish (Danio rerio) was performed in accordance with previous methods (Yang et al., 2021, 2023). Embryos were collected by spawning which was stimulated by setting of light within 30 min. At 7–9 h post fertilization (hpf), 15 embryos were randomly transferred to each wells of 12 well plates containing embryo medium with 250 µg/mL GSP and FSP.
Measurement of cell death and NO production in zebrafish larvae was conducted according to the previous method described by Yang et al. (2023). Briefly, zebrafish larvae treated with 250 µg/mL of GSP and FSP at 7–9 hpf, and then determine cell death and NO production using 10 µg/mL acridine orange (30 min) and 10 µM DAF-FM-DA (2 h) at 3 days post fertilization (dpf).
All the experiments were statistically analyzed using one-way ANOVA and Dunnett’s multiple comparison test in GraphPad Prism 8 (GraphPad Software, Sand Diego, CA, USA). Data were presented as mean ± SE. P-values of less than 0.05 (p < 0.05), 0.01 (p < 0.01) and 0.001 (p < 0.001) were considered significant.
Results
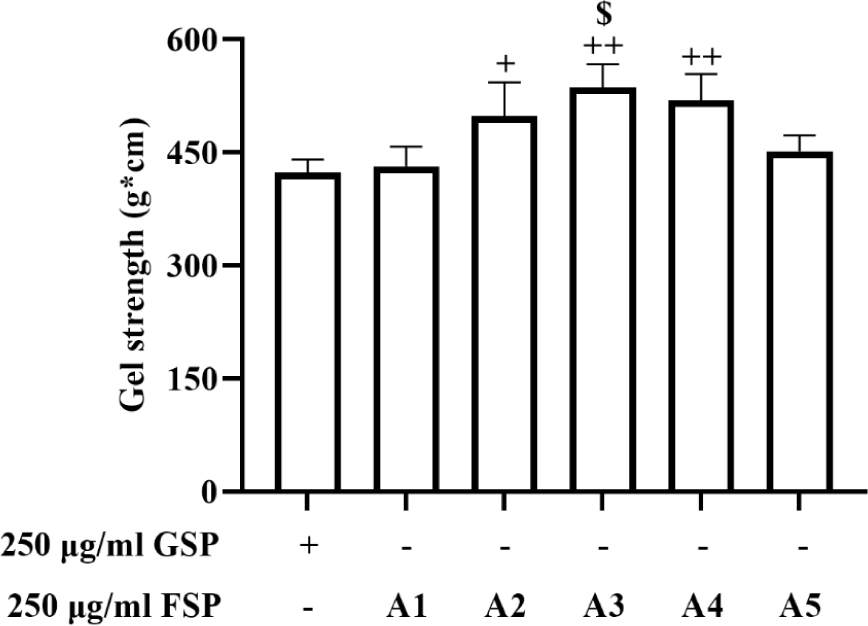
Gel strength, an inherent structural feature, analyses textural characteristics of gelatinous food (Park & Lin, 2005; Zhang et al., 2014). We investigate the change of texture in surimi with/without A. vera gel powder and Red ginseng extract using rheometer. As shown in Fig. 1, the gel strengths of FSP (A2, A3, and A4) were significantly higher than that of GSP. However, there was no significant difference among the GSP and FSP (A1 and A5). According to this result, we suggested that the gel strengths of surimi can be promoted by the additives, A. vera gel powder and Red ginseng extract.
As cell protection against oxidative stress is an important response in antioxidant effect (Burdon et al., 1989), we examined the effect of FSPs on H2O2-stimulated cell damage in Vero cells. Initially, to evaluate the cytotoxicity of GSP and FSP, the cells were treated with 125, 250, and 500 µg/mL of GSP and FSP for 24 h. As shown in Fig. 2A, the cell viability did not show a significant difference compared to the control group. Thus, 500 µg/mL of GSP and FSPs was determined as the optimum concentration for subsequent experiments.
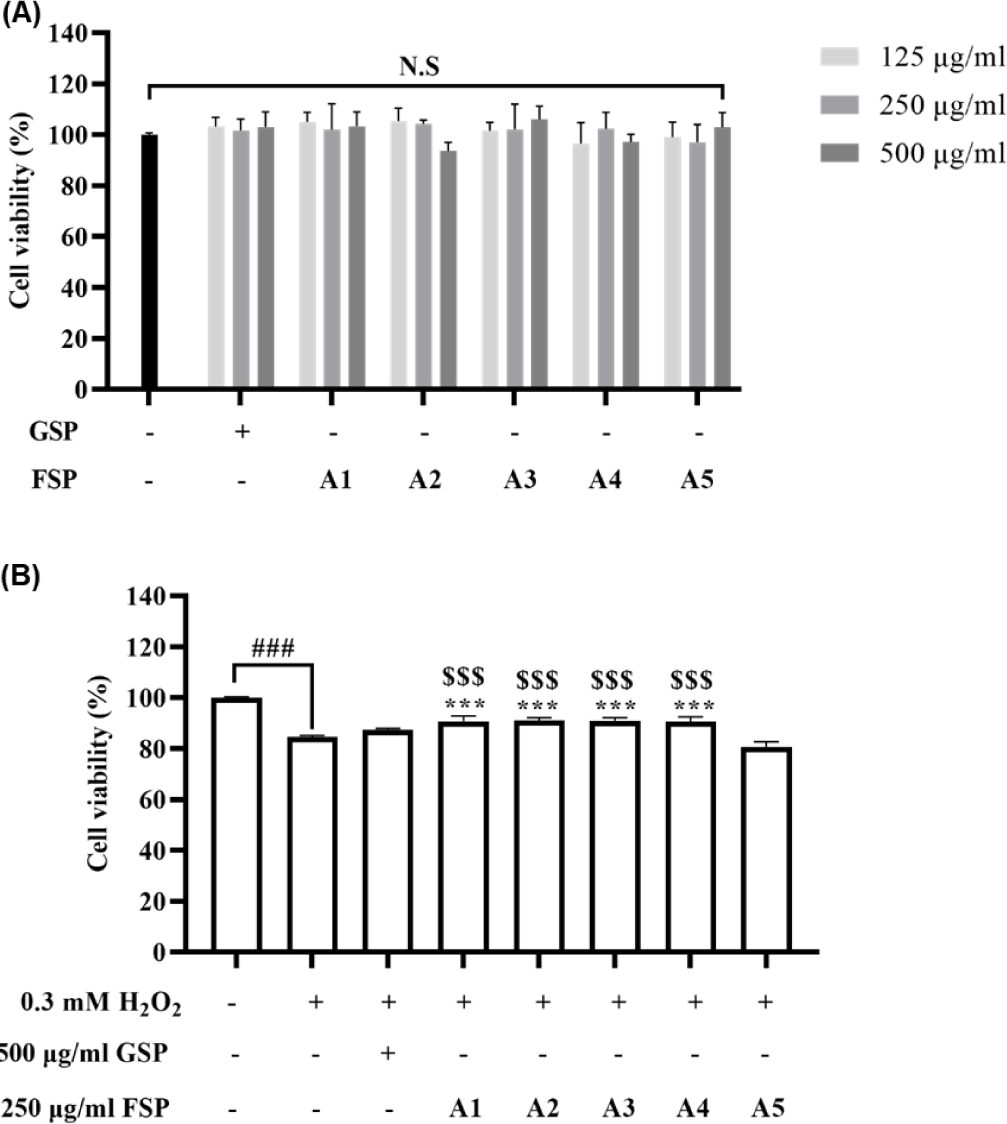
In addition, the treatment of 0.3 mM H2O2 into the cells led to decreasing cell viability, but FSPs treatments increased. In particular, in added functional ingredients groups (A1–A4), those groups led to a significant increase compared to that in the A5 group (Fig. 2B). These data suggest that the FSP containing A. vera gel powder only as well as the combination of them can induce the synergistic effect in cell protection against oxidative stress.
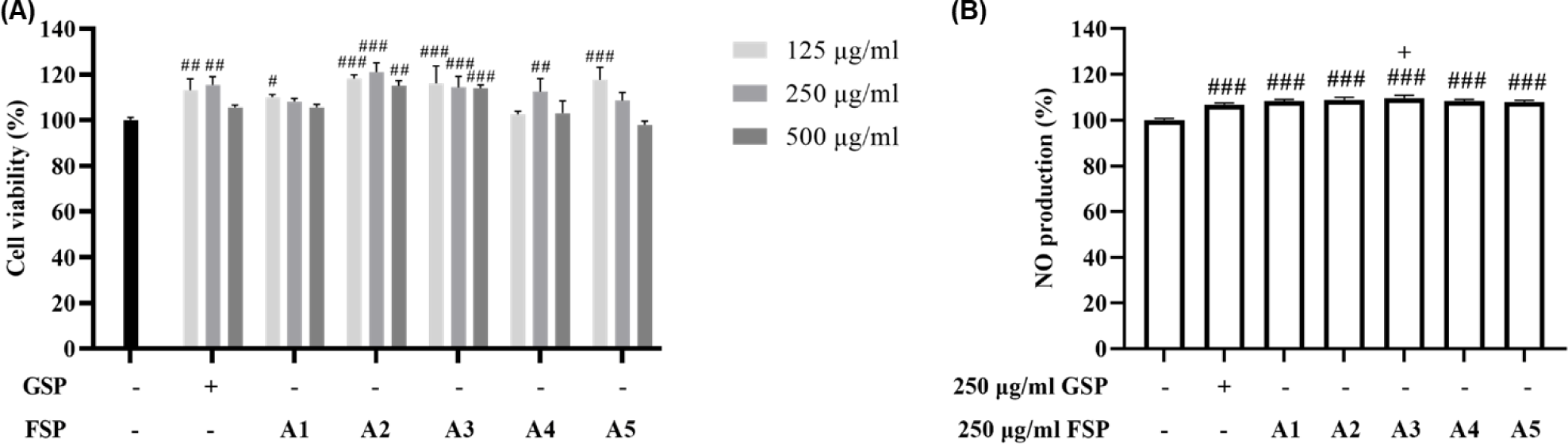
The immune response is sensitive to oxidative damage that can lead to a reduction in the numbers of specific receptors on immune cell surfaces (Hughes, 2000). To assess whether immune response is influenced by FSPs treatment which have cell protection effect against oxidative stress, we examined NO production in RAW264.7 cells by Griess assay. Initially, the cells were treated with 125, 250, and 500 µg/mL of GSP and FSP for 24 h, and cell viability was assessed using an MTT assay. There was no significantly decreased compared to those in the normal group (Fig. 3A). We determined the safe concentration range (125, 250, and 500 µg/mL) and selected 500 µg/mL as nontoxic concentration for subsequent experiments. As shown in Fig. 3B, GSP and FSP significantly increased NO production compared to those of the control group. It means that surimi products might enhance immunity. In particular, A3 of FSPs the highest significantly increased NO production among all the surimi products. These findings suggest that a particular combination of FSP between A. vera gel and Red ginseng extract could be used to produce an immune-enhancing surimi products in functional labeling food industry.
Zebrafish are considered as in vivo alternative with painless and used for bioactivities screening and drug development (Watzke et al., 2007; Yang et al., 2023). Given that oxidative damage is associated immune response (Hughes, 2000), we postulated that FSPs might engage immunomodulatory activity by cell death and NO production. Firstly, we evaluated the toxicity of various concentrations of GSP and FSPs by monitoring the survival rate of zebrafish embryos and larvae. After exposed up to 7 dpf, the survival rate did not show a significant difference between 125 and 250 µg/mL GSP and FSP compared to those of the control group (Fig. 4A). Whereas the survival gradually decreased 500 µg/mL GSP and FSPs. Thus, we selected 250 µg/mL as the optimum concentration for the subsequent experiments.
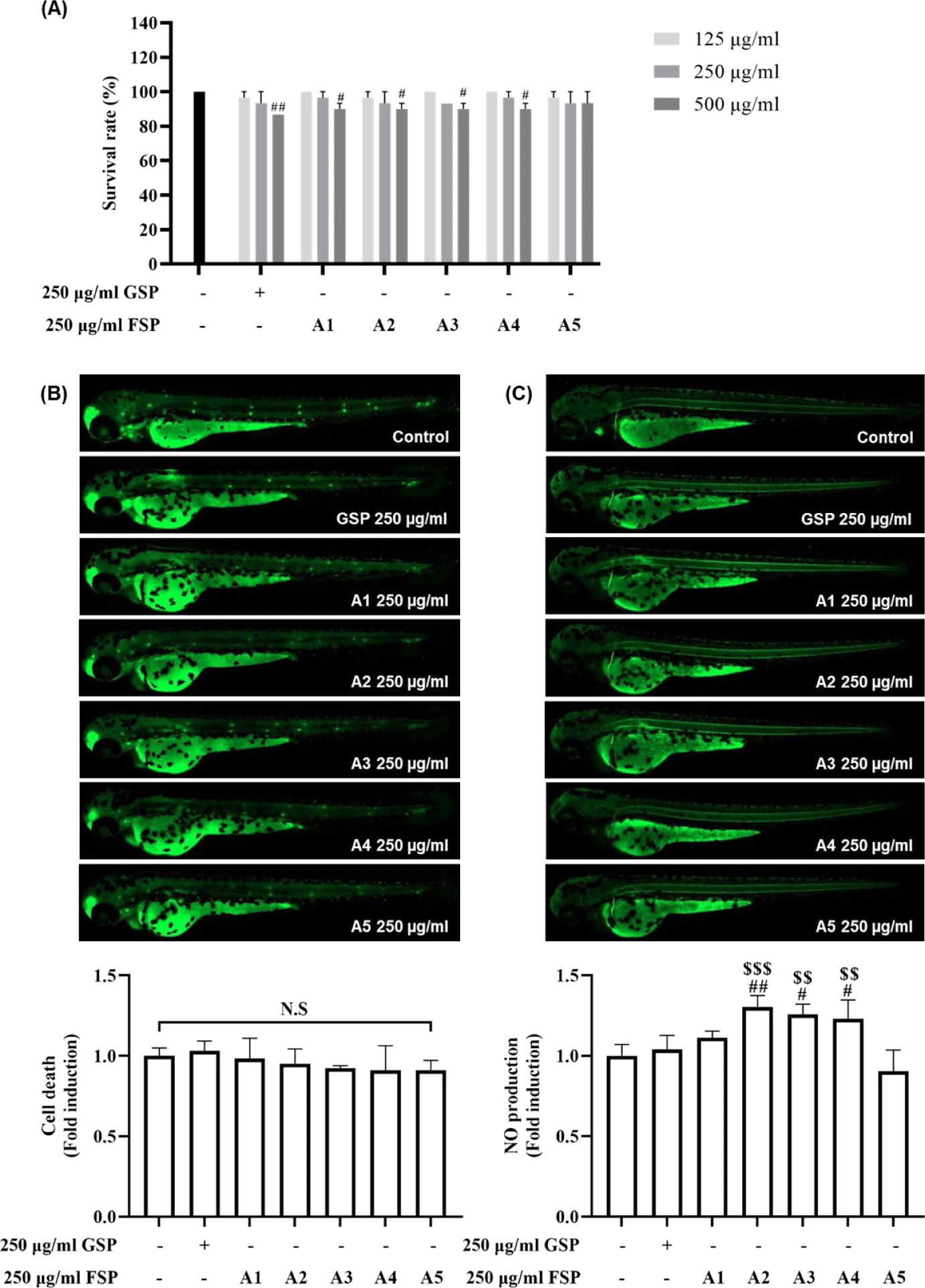
To examine whether FSPs lead to increased cell death, zebrafish larvae were stained with acridine orange for 30 min. As shown in Fig. 4B, there was no significant differences in fluorescence intensity compared to those of the control group. In addition, we evaluated the immune response of FSPs through stained with DAF-FM-DA for 2 h to stain NO production. In Fig. 4C, the fluorescence intensities were significantly increased in FSPs (A2, A3, and A4) compared to the control group, but there was no significant difference among the GSP and FSP (A1 and A5). This result implies that the combinations of the two functional ingredients led to significant increases, compared to those in the non-combined FSPs. These results indicated that the synergistic effect of the two functional ingredients in FSPs was proven for immune-enhancement in zebrafish larvae.
Discussion
During the emergence of COVID-19, the demand for functional ingredients continues to expand in response to an interest for life-enhancing benefits from their food. The Ministry of Food and Drug Safety (MFDS) allowed functional labeling for general foods using functional ingredients which have been scientifically proven to have certain functionality and no health issues such as taken of large quantities (Hamulka et al., 2020; MAFRA, 2021). In addition, previous study has reported that development of functional ingredients can be promote the functional labeling foods market in food industry (Choi, 2022).
Surimi as a seafood is functional food ingredient made from refined fish myofibrillar proteins and used to the processed foods as known common items such as eomook, fish balls, kamaboko, and imitation crab meat (Park, 2000; Xiong et al., 2009). Previous studies have evaluated the effects of gel properties and flavor characteristics from surimi and surimi product which contain bioactive ingredients (Sharma et al., 2023; Walayat et al., 2022). In addition, it was reported that surimi products such as eomook improves protective effects against oxidative stress. In the study, therefore, it was demonstrated that the synergistic effect of surimi product on immune response using artificial digestion system in in vitro and in vivo zebrafish model.
Generally, gel strength is a great impact on texture, tissue characteristics, water holding capacity and quality of surimi products (Park & Lin, 2005). As shown in Table 1, the two types of surimi products depending with/without the ratios of A. vera gel powder and Red ginseng extract between 0.4:0.0 to 0.0:0.4 were prepared, and referred as GSP and FSP. FSPs contained both A. vera and Red ginseng extract increased the gel strengths, suggesting the surimi products might be increased in gel strength by adding those functional ingredients.
In vitro digestion methods are adopted as tools to evaluate cell viability and immunity in vitro and in vivo zebrafish model (Oh et al., 2019, 2022). In addition, Tonon & Grassi reported that zebrafish could be potentially considered as a first-line testing model whose researcher could reduce the use of more complex and costly animal models (Tonon & Grassi, 2023). From the results, it was well proven that FSPs could protect the cell damage and increased NO production by combination ratios in in vitro and in vivo zebrafish model. We confirmed that FSP improved immune-enhancement with the combination mixtures of the two functional ingredients in surimi products. This results could be applied to a functional labeling food industry.
