Introduction
The aquaculture industry is growing rapidly in response to increased demand for human consumption. Intensive aquaculture farming systems have been introduced to maintain aquaculture products because of a decline of fish species in natural resources. However, the production of fish under intensive culture processes can lead to the consequent increase of stress and susceptibility to several infectious diseases as well as to immunosuppression (Hamid et al., 2021). To decrease excessive economic loss, several antibiotics and synthetic drugs are generally used in aquaculture production to prevent outbreaks of infectious disease agents and to improve growth of the fish (Munglue et al., 2020). Nevertheless, the use of drugs both in diets and in water treatments has caused drug-resistant strains, environmental contamination, and contaminated foods (Doan et al., 2019). Furthermore, numerous countries have maintained the prohibition on fish and their products that have been treated with antibiotics and other substances. In light of these factors, scientists have endeavored to discover innovative natural substances that have the ability to improve the development performance and overall health of fish (Farag et al., 2022; Panase et al., 2018a).
One such method is the development of various medicinal plant species as natural feed additives to increase fish growth and prevent infectious diseases (Jankham et al., 2020; Khorshidi et al., 2022). Medicinal plants have been used to treat a variety of illnesses in both humans and animals. Research indicates that several plant species have the potential to replace chemical medications in aquaculture due to their ability to promote fish health and growth (Wangkahart et al., 2022). For example, Panase et al. (2018a) found that supplementing hybrid catfish (Clarias macrocephalus × C. gariepinus) with 300 mg of Euphorbia hirta leaf extract improved their growth, hematological parameters, and organosomatic indices. Furthermore, hybrid catfish fed diets containing Houttuynia cordata Thunb. leaf extract demonstrated significant increases in growth, feed consumption, and hematological indices (Panase et al., 2018b).
Boesenbergia rotunda (L.) Mansf. is a perennial herb from the Zingiberaceae family. This plant is also known as Fingerroot or Chinese Key in English, Krachai or Krachai-Dang in Thai, and Temu Kunci in Malay (Eng-Chong et al., 2012). In many Asian nations, the plant is widely grown and consumed as a spice, flavoring ingredient, and herb (Ongwisespaiboon & Jiraungkoorskul, 2017). Traditional medicine has used it to treat allergies, ulcers, gastrointestinal diseases, dysmenorrhea, flatulence, and rheumatism (Eng-Chong et al., 2012). Many active chemicals from B. rotunda have been found, including alpinetin, pinostrombin, pinocembrin, cardamonin, panduratin, kaempferol, and quercetin (Eng-Chong et al., 2012). B. rotunda has been found in pharmacological investigations to have antibacterial, antiparasitic, antioxidant, antimutagenic, antifungal, antiviral, antiinflammatory, and anticancer properties (Eng-Chong et al., 2012). A recent study by Doan et al. (2019) found that Nile tilapia (Oreochromis niloticus) fingerlings fed diets enriched with B. rotunda powder for 8 weeks showed a substantial boost in growth performance, mucosal and serum immunological markers as compared to the control fish. Cardamonin, a phenolic compound obtained from B. rotunda extract, effectively enhanced the growth and intestinal structure of hybrid catfish when fed diets, in comparison to the control group (Khunchalee & Munglue, 2020).
The hybrid catfish was produced by crossing male African catfish, C. gariepinus, with female Thai walking catfish, C. macrocephalus (Senanan et al., 2004). This hybrid fish species is often cultivated throughout Thailand and is considered one of the most economically valuable freshwater fish in this country. The hybrid catfish has many commercially desirable traits such as rapid growth rate, tolerance to disease and low oxygen, and good flesh quality. Under intensive cultivation, this fish species is likely to be challenged by slow growth and the prevalence of outbreaks of numerous infectious diseases (Jankham et al., 2020).
As previously stated, B. rotunda is commonly used in folk medicine to treat a variety of human diseases. However, the application of B. rotunda as a feed addition in aquaculture remains limited, and more information on its favorable effects on fish growth and physiological aspects is needed (Doan et al., 2019). Therefore, the objective of this research was to assess the effects of B. rotunda rhizome extract on growth performance, intestinal histology, digestive enzymes, hematological, and biochemical indicators in hybrid catfish.
Materials and Methods
The B. rotunda rhizome samples were collected from a garden in Khueang Nai District, Ubon Ratchathani Province, Thailand. A botanist authenticated a plant specimen, and the voucher specimens were stored in the Biology program of the Faculty of Science at Ubon Ratchathani Rajabhat University. The rhizomes were rinsed with flowing tap water, then sliced into small fragments measuring roughly 2–3 mm, and subsequently dehydrated in a hot air oven (UN260, Memmert GmbH, Buchenbach, Germany) at a temperature of 40°C for a duration of 48 hours. The samples (2 kg) were pulverized into a fine powder and subjected to extraction using 70% methanol (10 L) at a ratio of 1:5 (%w/v) for a duration of 1 week at room temperature. The extract was filtered using a Whatman filter paper No. 1. The solvent was removed through evaporation using a rotary evaporator (BÜCHI Rotavapor R-124, Marshall Scientific, Hampton, NH, USA) at a temperature range of 35°C–40°C, and the remaining substance was dried using a lyophilizer (Freeze-zone 12 plus, Labconco, Kansas City, MO, USA). Following the removal of the solvent, the resulting extract weighing 152.91 g was placed in amber glass bottles and kept in a refrigerator at –4°C until the experiment was conducted.
The B. rotunda rhizome extract was subjected to quantitative analysis using the procedures outlined by Abd’quadri-Abojukoro et al. (2022) and Otitolaiye et al. (2023) with slight adjustments. The total alkaloid content was quantified using a spectrophotometric technique, and the outcome was reported as milligrams of atropine equivalent per gram of extract (mg AE/g of extract). The flavonoid concentration was assessed with the aluminum colorimetric method and quantified as milligrams of quercetin equivalent per gram of extract (mg QE/g of extract). The quantification of phenolic components was conducted using the Folin-Ciocalteu colorimetric method and reported as milligrams of gallic acid equivalent per gram of extract (mg GAE/g of extract). The saponin level was quantified using the vanillin-sulphuric acid method and expressed as milligrams of diosgenin equivalent per gram of extract (mg DE/g of extract). The Folin-Ciocalteu colorimetric method was used to assess the total tannin concentration. The results were represented as milligrams of tannic acid equivalent per gram of extract (mg TAE/g of extract). The steroid concentration was evaluated using a spectrophotometric assay and reported as milligrams of diosgenin equivalent per gram of extract (mg DE/g of extract). The analysis of each phytochemical was conducted in triplicate using a microplate reader (SPECTRO Star Nano, BMG LabTech, Ortenberg, Germany).
The DPPH radical scavenging activity was determined following the protocol outlined by Sopitthummakhun et al. (2021), with certain adjustments. In summary, 1.6 mL of B. rotunda extract at various doses ranging from 0 to 1,000 µg/mL was combined with 0.1 mM DPPH. The solutions were left to react at room temperature for a duration of 30 minutes. The samples were analyzed for absorbance at a wavelength of 515 nm using a UV-visible spectrophotometer (Lambda 12, Perkin Elmer, Waltham, MA, USA), with a blank used as a reference. Ascorbic acid served as the standard reference. The DPPH radical scavenging activity (%) was determined using the following formula:
where Abcontrol is the absorbance of the control solution, and Absample is the absorbance of the sample solution or standard reference. The percentage of inhibition was then determined, and the IC50 value estimated. The experiment was carried out in triplicate at each concentration.
The basal diet (product name: Higade 9006T, floating pellets with a diameter of 1 mm) for the entire experimental trial was purchased from Charoen Pokphand Foods PCL, Bangkok, Thailand. The moisture (%), crude protein (%), crude lipid (%), and ash (%) contents of the basal diet were determined using the techniques provided by AOAC (2010). The gross energy (cal/g) was evaluated using bomb calorimetry. Table 1 shows the proximate composition of the diet. The dietary sample was crushed and sieved. Different amounts of B. rotunda extract powder (0 [control], 250, and 500 mg/kg diet) were combined to create a diet for testing, with cassava powder serving as a binder. The levels of B. rotunda rhizome extract used in this study were based on a publication by Sivaram et al. (2004), with minor adjustments. The diet samples were mixed with distilled water to make a dough, which was then pelleted using a floating fish feed extrusion machine. Pellets 1 mm in diameter were dried in a hot air oven at 40°C for 24 hours, then placed in polyethylene bags and stored at 4°C until the study was conducted.
| Analysis | Values |
|---|---|
| Moisture (%) | 7.35 |
| Crude protein (%) | 43.37 |
| Crude lipid (%) | 5.25 |
| Ash (%) | 10.68 |
| Calculated gross energy (cal/g) | 4,273.62 |
The fish were cared for in accordance with the protocols established by the Committee on Care and Use of Laboratory Animal Resources, National Research Council of Thailand. The Institutional Animal Care and Use Committee at Ubon Ratchathani Rajabhat University, Thailand, has formally accepted the experimental protocol AN63004.
Hybrid catfish fingerlings weighing 5.24 ± 0.26 g were obtained from Ubon Ratchathani Fishery Cooperative in Thailand and cultured in fiberglass tanks with continuous aeration. Fish were acclimatized in a lab for two weeks and fed commercial fish feed twice daily. Fish health, appearance, and feeding behavior were examined regularly.
A completely randomized design was used in this experiment. One hundred and eighty fish were randomly assigned to nine aerated circular concrete tanks (75 cm diameter and 30 cm water level) after acclimation. Fish were fed the basal diet or the basal diet supplemented with 200 and 500 mg B. rotunda/kg diet at 3% of body wet weight twice a day (8 a.m. and 4 p.m.) for 8 weeks. Aerated water was used to change one-third of each tank’s water every two days during the experiment. The following water quality was maintained during acclimatization and feeding: dissolved oxygen 5.7–6.5 mg/L, pH 7.21–7.82, temperature 25°C–28°C, alkalinity 25–100 mg/L, total hardness 50–100 mg/L, ammonia < 0.025 mg/L, nitrite < 0.02 mg/L, and nitrate 0.1–4.5 mg/L. These water quality parameters indicated suitable conditions for the culture of fish. The experiment used natural light and dark cycles.
At the end of the trial, fish in each tank were not fed for 24 h, counted, and weighed. Fish growth performance characteristics were estimated using these formulae.
Intestinal samples were obtained from 3 fish per replication (9 fish per treatment) and separated into three sections: proximal, middle, and distal. The intestinal lumen of each portion obtained from each fish was cleansed with PBS buffer (Sigma-Aldrich, St. Louis, MO, USA), pH 7.4, fixed in 10% neutral buffered formalin, dehydrated in alcohol, and cleared in xylene. The slices were immersed in paraffin wax, then sliced into 5 µm ribbons with a microtome and placed on microscope slides. The sections were stained with hematoxylin and eosin (H&E). The approach described by Escaffre et al. (2007) was used to assess villi height (VH), villi width (VW), absorptive area, inner circular smooth muscle (ICSM), outer longitudinal smooth muscle (OLSM), and goblet cells of the gut.
The proximal intestine samples were obtained from 3 fish each replication (9 fish per treatment). Each sample was homogenized in a solution of 10 volumes (w/v) of cold 0.9% normal saline individually. The homogenized samples were then centrifuged at 5,000×g for 20 minutes at 4°C. The supernatant was collected and kept at –20°C until the activity of digestive enzymes was determined. The total protein content was calculated using the Lowry technique (Lowry et al., 1951). The activity of amylase was tested using 1% starch solution as a substrate (Thongprajukaew et al., 2010). The reaction was measured at 540 nm and compared to the maltose standard curve given by Thongprajukaew et al. (2010). Protease activity was tested using 1% azocasein as a substrate and measured at 440 nm against a tyrosine standard curve (Cupp-Enyard, 2008). Lipase activity was measured with 0.01 M p-nitrophenyl palmitate as a substrate (Thongprajukaew et al., 2010). The reaction was approximated at 410 nm using a standard curve for p-nitrophenol. Digestive enzyme activity was expressed in units per milligram of protein.
At the end of the experiment, 3 fish from each tank were randomly selected (9 fish per treatment). Each fish was anesthetized in a bath containing 5 mL/L clove oil. Fish were carefully examined for deep anesthesia. Blood samples (1 mL) were taken from the caudal vein and separated into two aliquots. One aliquot (0.5 mL) was transferred to tubes containing sodium heparinate as an anticoagulant and used in the hematological assay. The remaining portion (0.5 mL) was gently transferred into anticoagulant-free tubes, allowed to clot at 4°C, and centrifuged at 5,000×g for 10 minutes at 25°C to extract serum for blood biochemical examination.
A Neubauer hemacytometer was used to count white and red blood cells (WBC and RBC). The hemoglobin level was determined using the cyanohemoglobin technique. To determine hematocrit content, a microhematocrit tube was filled with heparinized blood and centrifuged at 15,000×g for 5 minutes.
The levels of aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatinine, glucose, triglycerides, cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), albumin, total bilirubin (T-bilirubin), and direct bilirubin (D-bilirubin) were measured using the protocols described by Jankham et al. (2020). The total protein content was measured using the Lowry technique (Lowry et al., 1951).
The Kolmogorov-Smirnov test and Levene’s test were employed to assess data normality and variance homogeneity, respectively. The results are reported as mean ± SEM. Statistical differences between treatments were evaluated using one-way analysis of variance (ANOVA), followed by Duncan’s Multiple Range Test (DMRT). A significant difference was considered at p < 0.05.
Results
Table 2 shows the phytochemical and DPPH radical-scavenging activities of B. rotunda extract. The presence of secondary metabolites of B. rotunda extract was observed at high levels in the following: phenolics (543.86 ± 1.17 mg GAE/g of extract), saponins (478.42 ± 3.62 mg DE/g of extract), steroids (209.81 ± 2.80 mg DE/g of extract), flavonoids (156.12 ± 0.48 mg QE/g of extract), alkaloids (77.63 ± 3.10 mg AE/g of extract), and tannins (23.17 ± 0.27 mg TAE/g of extract). Antioxidant activity of B. rotunda rhizome extract evaluated by using the DPPH assay elicited an IC50 value of 268.89 ± 1.95 µg/mL, whereas ascorbic acid had an IC50 value of 66.52 ± 0.32 µg/mL.
Table 3 summarizes the effects of dietary supplementation with B. rotunda extract on hybrid catfish growth. Fish fed with experimental diets showed significantly higher growth parameters (final weight, WG, SGR, and ADG) compared to the control diet (p < 0.05). Fish fed with B. rotunda extract diets showed a substantial decrease in FCR compared to the control group (p < 0.05). There was no significant difference in SR between treatments (p > 0.05).
Table 4 shows the effects of B. rotunda extract supplementation on the intestinal histology of hybrid catfish. In the proximal gut (Fig. 1A–1C), fish fed diets supplemented with B. rotunda extract at 250 mg/kg showed a substantial increase in VH compared to the other diets (p < 0.05). Increased VW, absorptive area, and goblet cells were identified in fish fed with the experimental diets compared with the control (p < 0.05). Fish fed a 500 mg/kg diet had the greatest ICSM value compared to other groups (p < 0.05). The OLSM showed no significant differences between the treatments (p > 0.05).
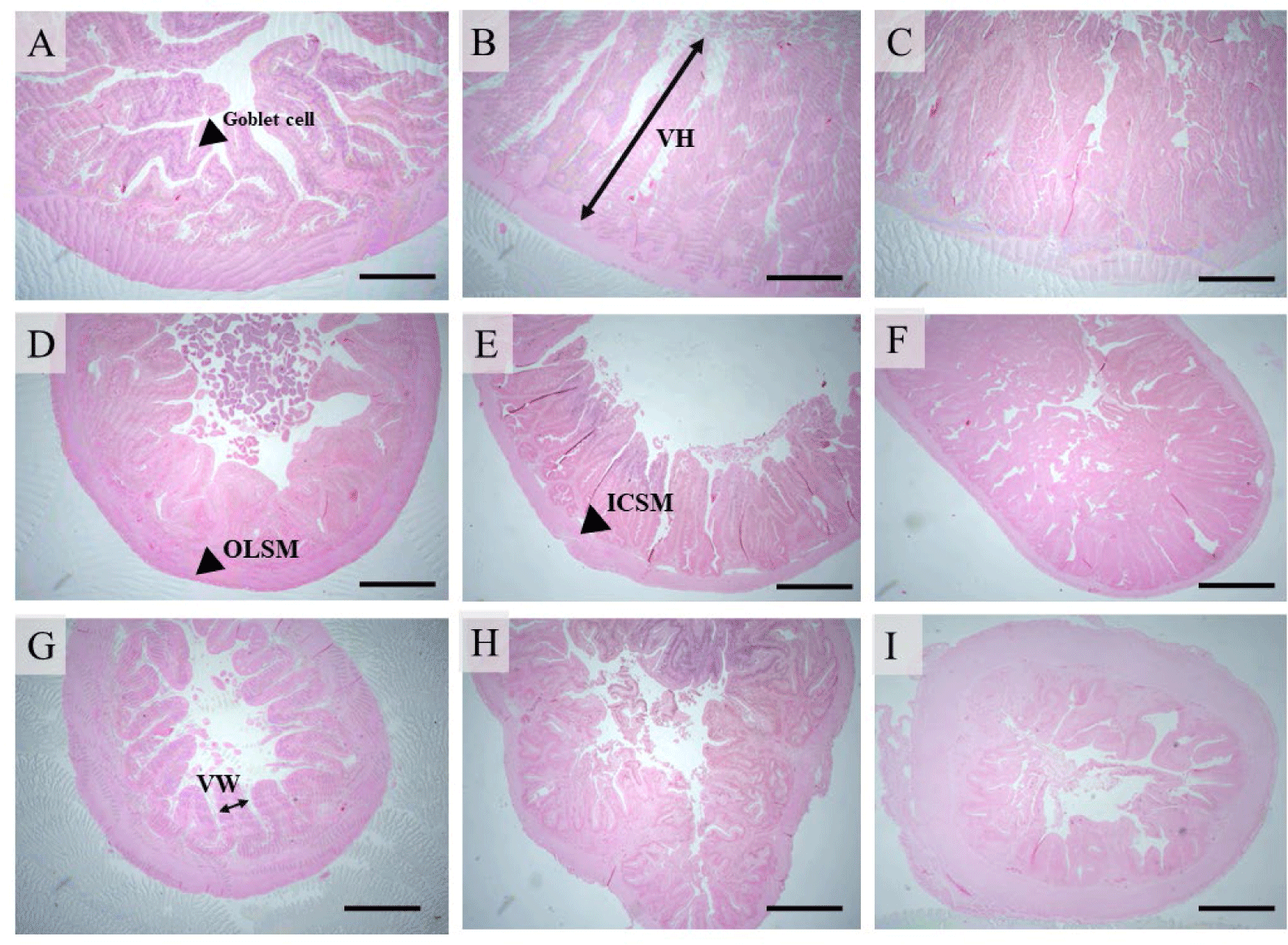
In the middle intestine (Fig. 1D–1F), fish fed diets supplemented with B. rotunda extract had significantly higher levels of VH, VW, ICMS, OLSM, and goblet cell compared to the control (p < 0.05). Fish treated with 250 mg B. rotunda/kg diet showed higher absorptive area than other groups (p < 0.05).
The VH in the distal intestine (Fig. 1G–1I) did not differ significantly between treatments (p > 0.05). Fish fed a diet supplemented with 250 mg B. rotunda/kg had the highest VW and absorptive area values compared to other diets (p < 0.05). Treatments fed B. rotunda diets showed significantly higher ICSM and OLSM layers than the control group (p < 0.05). Fish treated with 500 mg B. rotunda/kg diet had considerably more goblet cells compared to other groups (p < 0.05).
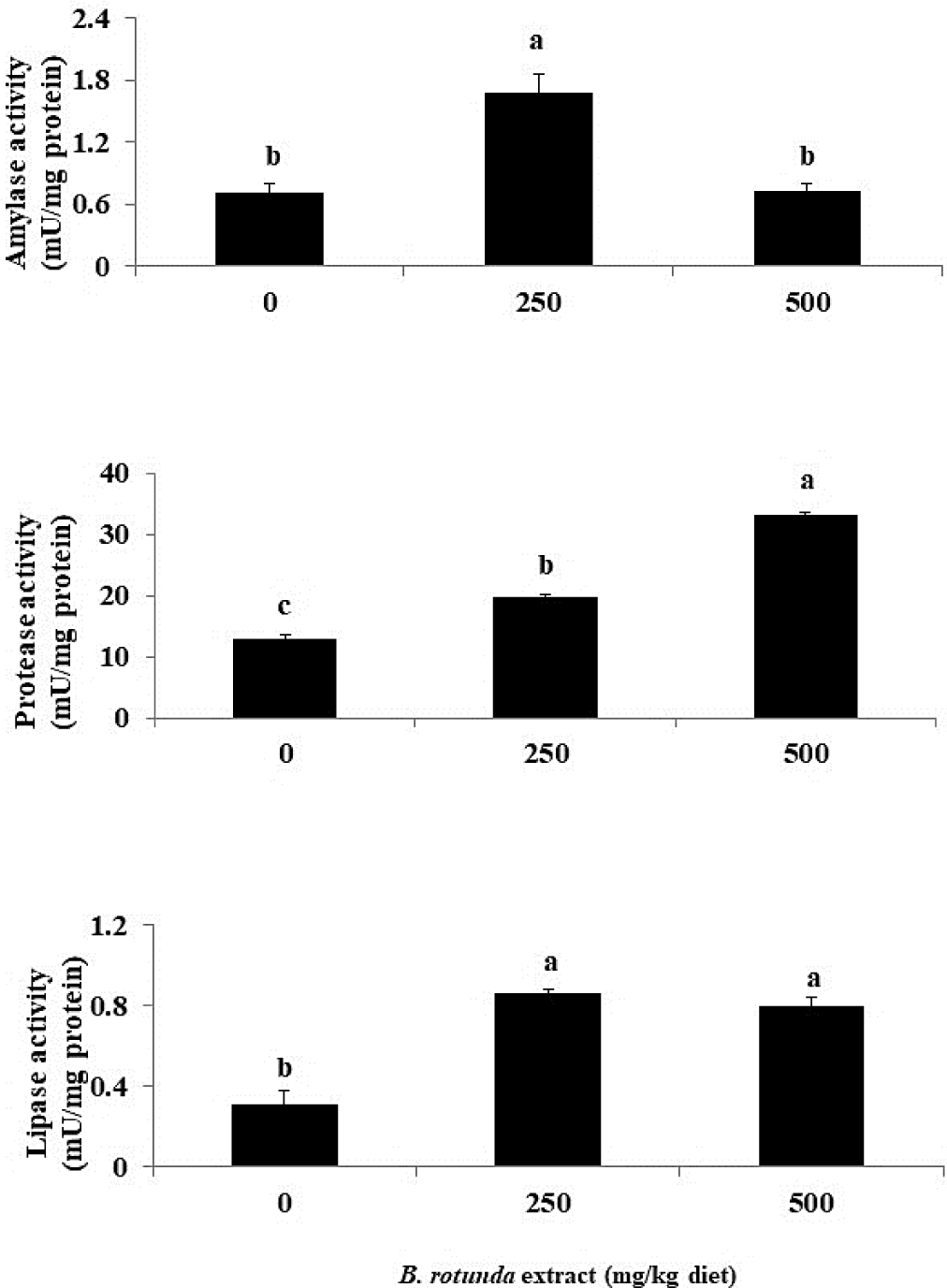
The amylase activity of fish that were fed a diet containing 250 mg B. rotunda was significantly higher than that of the other groups (p < 0.05), as illustrated in Fig. 2. Furthermore, fish that were provided with diets containing B. rotunda exhibited a substantial increase in protease and lipase activities in comparison to the control diet (p < 0.05).
Compared to the control fish, the concentrations of WBC, RBC, hemoglobin, and hematocrit were significantly higher in fish fed diets supplemented with B. rotunda (p < 0.05, Table 5).
The effects of dietary B. rotunda treatment on blood biochemical markers in hybrid catfish are presented in Table 6. Fish fed diets supplemented with B. rotunda extract had considerably higher blood glucose levels compared to those fed the basic diet (p <0.05). Fish fed with the experimental diets showed significantly lower cholesterol levels compared to the control group (p < 0.05). There were no significant differences in serum AST, ALP, total protein, albumin, creatinine, triglyceride, HDL-C, LDL-C, T-bilirubin, and D-bilirubin levels between fish given the studied diets and those fed the control diet (p > 0.05).
Discussion
In a recent study, quantitative determination of B. rotunda extract was shown to contain various levels of alkaloids, flavonoids, phenolics, saponins, tannins, and steroids (Ongwisespaiboon & Jiraungkoorskul, 2017). Phenolics had the highest level compared to other constituents followed by saponins, steroids, flavonoids, and alkaloids, respectively. The extract had only trace levels of tannins. Oxidative stress occurs when fish are exposed to an excessive number of free radicals (Xie et al., 2017). Medicinal herbs with antioxidant properties can be utilized as dietary additives to protect cultured fish against oxidative stress and other infectious diseases (Safari et al., 2020; Wangkahart et al., 2022). By using the DPPH assay, the plant extract had an IC50 value of 268.89 ± 1.95 µg/mL. However, the IC50 value produced by B. rotunda extract was lower than that of ascorbic acid (66.52 ± 0.32 µg/mL), a standard reference. Previous studies suggested that the growth-promoting activities of medicinal plants in aquatic animals could be attributed to the actions of phytochemicals such as alkaloids, flavonoids, phenolics, tannins, and steroids (Midhun et al., 2016; Vijayaram et al., 2022). Furthermore, flavonoids, phenolics, and tannins are known to have antioxidant, anti-inflammatory, and antimicrobial effects (Otitolaiye et al., 2023). In this regard, it can be presumed that B. rotunda extract could be used as natural growth enhancer and antioxidant in aquafeed.
The current investigation observed enhanced growth parameters in fish that were provided with diets supplemented with B. rotunda for a duration of 8 weeks. In comparison to the basal diet, dietary B. rotunda significantly decreased the FCR of hybrid catfish. Similary, research has shown that supplementing Nile tilapia figerlings with dietary B. rotunda powder significantly increased their growth parameters in comparison to the control group (Doan et al., 2019). Furthermore, in comparison to the control fish, rainbow trout (Oncorhynchus mykiss) that were fed diets supplemented with Zingiber officinale rhizome extract exhibited substantial gains in ultimate weight, WG, and feed intake (Shaluei et al., 2016). The enhancement of appetite, digestive enzyme activities, and metabolic processes has been widely recognized as potential mechanisms by which medicinal plants or their active compounds promote growth in fish (Farag et al., 2022; Khorshidi et al., 2022; Vijayaram et al., 2022). Prior studies have documented the potential of secondary metabolites, including phenolics, alkaloids, and flavonoids, to enhance the growth of fish (Vijayaram et al., 2022). Midhun et al. (2016) discovered that the addition of curcumin into the diets of Nile tilapia resulted in a notable upregulation of the expression levels of GH and IGF genes. In comparison to the control fish, the expression of GH and IGF genes was significantly upregulated in Beluga sturgeon fed diets supplemented with polyphenols (Safari et al., 2020). A potential explanation for the enhanced growth performance observed in hybrid catfish through B. rotunda is that it improves digestive processes, nutrient utilization, and the palatability of fish diets (Wangkahart et al., 2022). The significant increase in fish growth parameters observed in the experimental groups may have been caused by the upregulation of growth-related gene expressions induced by B. rotunda supplementation (Ahmadifar et al., 2021). Interestingly, this research also found that catfish fed a 500 mg B. rotunda diet tend to have lower growth indices than fish fed a 250 mg B. rotunda diet. Phytochemical determination revealed that B. rotunda extract contains various anti-nutritional factors, such as tannins and saponins. These anti-nutritional components may impede fish growth and nutrient utilization by inhibiting digestive enzymes, absorption processes, or gut microbiota (Doan et al., 2019). However, additional investigation is required in order to elucidate the precise mechanism through which B. rotunda extract promotes growth in fish.
The digestive and absorptive functions of the digestive tract are associated with the intestinal morphology of fish. An increase in absorptive area, VH, VW, ICSM, OLSM, and goblet cells was observed in fish that were fed with B. rotunda in this investigation as opposed to fish that were fed the basal diet. By increasing intestinal VH, VW, and absorptive area, dietary B. rotunda could enhance the assimilation of nutrients in fish (Jankham et al., 2020). Goblet cells are of significant importance in the synthesis of mucin, an intestinal mucosa that prevents the entry of numerous pathogenic microorganisms into the intestinal wall (Munglue et al., 2020). Dietary B. rotunda increased the number of intestinal goblet cells in hybrid catfish, according to this study. Jankham et al. (2020) and Ahmadifar et al. (2021) hypothesize that bioactive compounds present in B. rotunda, including flavonoids and phenolics, might induce mitotic division in the crypt-villus region of the intestine, leading to a substantial augmentation of the VH. Also, antioxidant activity of B. rotunda can protect intestinal epithelial cells from inflammation and oxidative stress, hence maintaining intestinal integrity in fish (Khunchalee & Munglue, 2020). Therefore, the results of this study suggest that supplementing hybrid catfish with B. rotunda can enhance the histomorphometry of the intestinal tract.
The study revealed a notable enhancement in digestive enzyme activity among fish that were fed diets enriched with B. rotunda extract. Digestive enzymes are crucial for the process of breaking down vital nutrients in the digestive tract. Multiple studies have shown that incorporating medicinal plants into diets can enhance the functioning of pancreatic exocrine acini, leading to increased activity of digestive and absorptive enzymes (Khorshidi et al., 2022; Midhun et al., 2016; Wangkahart et al., 2022). Additionally, these plants can also regulate the microbial populations in the gut of fish (Vijayaram et al., 2022). This information is supported by the research conducted by Abd El-Hamid et al. (2021) and Khorshidi et al. (2022). The effects mentioned can be attributed to the actions of specific compounds, such as flavonoids and phenolics, found in the methanolic extract of B. rotunda. These compounds can aid in digestion by increasing the expression of genes related to digestive enzymes in fish (Midhun et al., 2016). The research findings indicate a positive correlation between the observed increase in digestive enzyme activity and improved growth and feed consumption efficiency. This study is the first reported evidence of B. rotunda enhancing digestive enzyme activity in hybrid catfish. Additional investigation is needed to assess the precise mechanism by which B. rotunda affects digestive enzyme activity in fish.
Hematological indices are frequently employed to assess the physiological reactions of fish when they are fed various substances in their diets (Abd El-Hamid et al., 2021; Panase et al., 2018a, 2018b). The current study has demonstrated a considerable rise in white blood cell levels in fish that were fed diets enriched with B. rotunda, in comparison to the control fish. The findings indicate that B. rotunda possesses immunostimulant properties in fish. The red blood cell count, hemoglobin concentration, and hematocrit level in fish that were fed diets containing B. rotunda were significantly higher compared to the control group. The observed elevation in hematological parameters in fish that were fed diets supplemented with functional additives may be attributed to an enhanced rate of proliferation and differentiation of hematopoietic stem cells, which is influenced by flavonoids, saponins, polyphenols, and other plant-derived chemicals (Dadashpour et al., 2017). Nevertheless, additional research is needed to fully understand the specific mechanism via which B. rotunda extract enhances the blood parameters in fish.
Blood biochemical measures have been utilized to assess the nutritional and physiological state of fish fed diets with herbs or phytochemicals (Adel et al., 2020). Serum glucose is a key source of energy in fish. According to Öner et al. (2008), fish subjected to stress had higher glucose levels. AST, ALP, total protein, albumin, T-bilirubin, and D-bilirubin are commonly used to assess liver function in fish (Jia et al., 2021). An increase in AST and ALP activity is associated with a higher risk of hepatocellular damage. Total protein content is linked to non-specific immunity (Jia et al., 2021). Albumin regulates colloid osmotic pressure, transports various chemicals, and protects against pathogens (Opute & Oboh, 2021). Triglyceride, cholesterol, HDL-C, and LDL-C levels are all implicated in liplid metabolism in fish (Jia et al., 2021). In the current investigation, fish fed diets containing B. rotunda showed elevated serum glucose levels. Presumably, a rise in blood glucose levels after feeding with a B. rotunda diet may indicate a higher energy need for fish to maintain homeostasis under stress through glucogenesis and glycogenolysis (Iwama et al., 1999; Öner et al., 2008). Furthermore, higher glucose levels in fish could suggest a changed carbohydrate metabolism associated with the supplement, nutritional status, intestinal absorption, and experimental circumstances (Iwama et al., 1999). Thus, additional study regarding the effect of B. rotunda on fish blood glucose concentrations is strongly encouraged. Dietary supplementation with B. rotunda extract resulted in a significant decrease in serum cholesterol levels in hybrid catfish compared to fish fed a basal diet. The observed decrease in serum cholesterol levels in the studied fish may be attributed to the hypolipidemic effect of B. rotunda, which can prevent the accumulation of fat in the liver (Farag et al., 2022). This study also found that the levels of AST, ALP, total protein, albumin, creatinine, triglyceride, HDL-C, LDL-C, T-bilirubin, and D-bilirubin in fish fed B. rotunda supplemented diets were comparable to those in fish fed the control diet. Therefore, our data may validate the use of B. rotunda rhizome extract as a feed additive with no negative effects on hybrid catfish.
Conclusion
In summary, this research reveals that B. rotunda rhizome extract supplementation enhanced growth performance and physiological variables in hybrid catfish. The growth-enhancing effect of B. rotunda in fish can be related to the presence of alkaloids, flavonoids, phenolics, saponins, tannins, and steroids compounds. Antioxidant activity of B. rotunda could also support the overall health and well-being of hybrid catfish. Dietary supplementation of B. rotunda at 250 mg/kg diet is recommended for hybrid catfish farming.
