Introduction
The black rockfish, Sebastes inermis, commonly known as black rockfish, is a marine fish belonging to the family Sebastidae. It is known to be a benthopelagic fish, meaning that it inhabits both the water column and seabed and is primarily found in nearshore rocky habitats (Kim et al., 2005; NFRDI, 2004; Plaza et al., 2004). To date, approximately 110 species of Sebastes have been reported worldwide (Kai et al., 2003; Nelson et al., 2016), of which 25 have been reported in Korea (MABIK, 2023). Most of these species are commercially important. Black rockfish are a major target species for coastal fisheries and have been included in marine ranching projects in the South Sea over the past 20 years. Consequently, continuous seed release is being carried out (MOMAF, 2004, 2006; NFRDI, 2007).
Studies on fish feeding ecology are crucial for understanding and interpreting the food web structure of marine ecosystems. They provide valuable biological information that can be used to understand the feeding characteristics, activities, and growth of various fish species (Akeda et al., 2012; Carter et al., 1991; Linke et al., 2001). Additionally, research on fish feeding ecology can be used to gain a clearer picture of the feeding characteristics and nutritional development stages of commercially important species, and to develop resource-ecological models (Jobling, 1982; Lied & Braaten, 1984). Previous studies on the feeding ecology of Sebastes species have shown that those inhabiting eelgrass beds and rocky areas primarily consume benthic organisms such as amphipods, copepods, and macrurans (Akeda et al., 2012; Huh & Kwak, 1998a; Kim & Kang, 1999). Other studies have reported that Sebastes schlegelii mainly feeds on Brachyura, Macrura, and fish (Park et al., 2007; Seo & Hong, 2007). While a previous study by Kim et al. (2009) examined the dietary changes in released S. inermis in the submerged area of Jinhae Bay based on their total length (TL), this study focused only on individuals smaller than 10 cm. Therefore, it is likely that S. inermis in the Yeosu coastal area also utilizes benthic animals as their primary food source.
This study aims to accumulate biological information through a comparative analysis of the feeding habits of S. inermis from various perspectives, considering that the feeding characteristics of S. inermis may vary depending on its geographical location and growth stage, even within the same species and habitat.
Materials and Methods
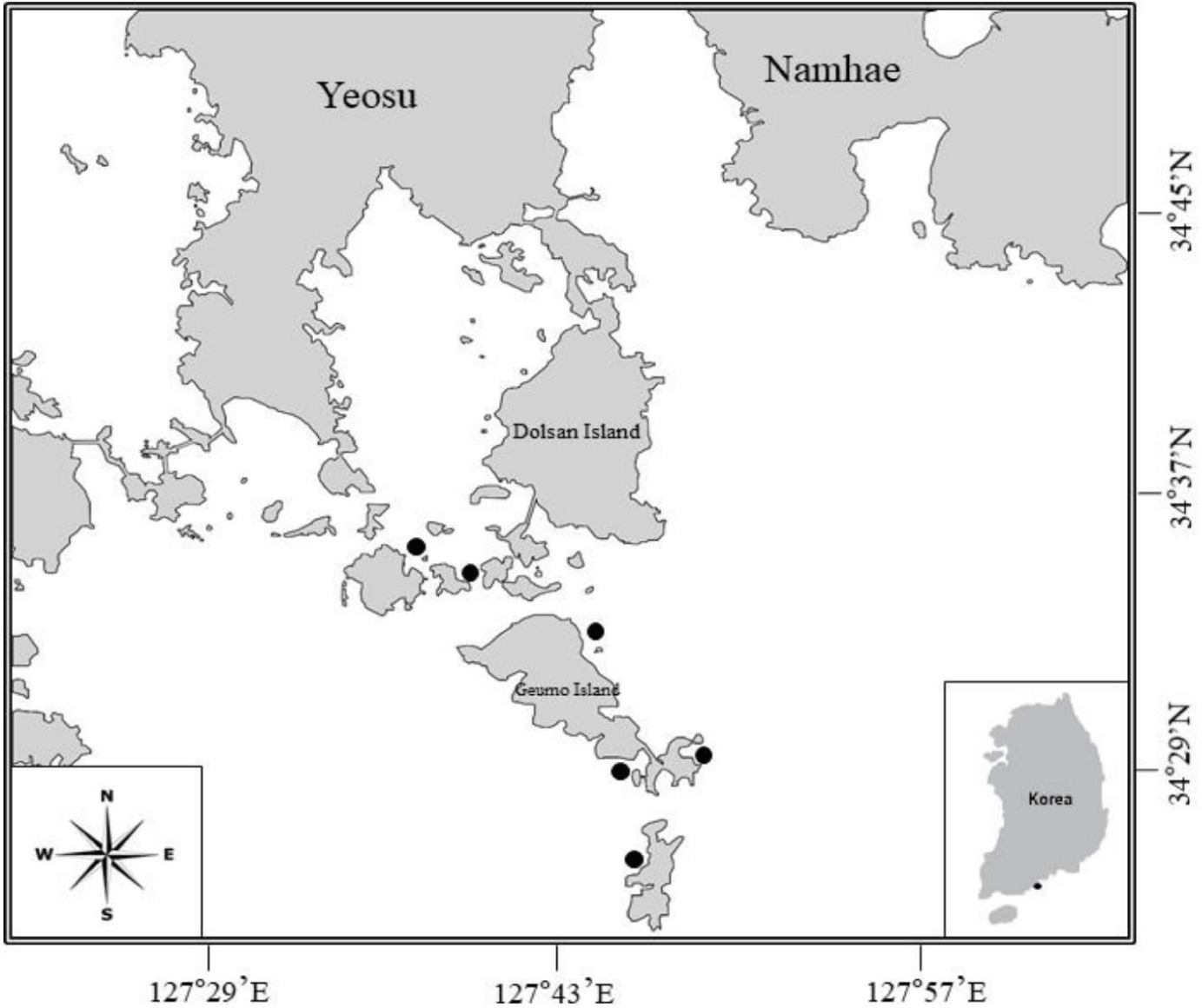
The black rockfish S. inermis specimens used in this study were procured by the South Sea Fisheries Research Institute, National Institute of Fisheries Science, from the coastal waters of Yeosu City, Jeollanam-do Province, Korea (including Gaedo, Geumodo, and Yeondo Islands) using gill and fyke nets from June 2009 to May 2010 (Fig. 1). For each sample, the TL was measured and relevant data, such as collection date and gear type, were recorded. Subsequently, the stomachs were dissected and preserved in 100 mL vials filled with neutral formalin until analysis.
To evaluate the dietary habits of S. inermis, stomach samples were rinsed with distilled water and dissected to allow content examination. Prey organisms retrieved from stomach contents were meticulously identified to the species level using a dissecting microscope (SZX16, Olympus, Tokyo, Japan), as described by Kim et al. (2005) for fish and Hong et al. (2006) for invertebrates. Prey organisms that posed challenges for species identification were classified to higher taxonomic levels. After identification, the wet weight of each prey organism was measured using an analytical balance (XP26, Mettler Toledo, Shanghai, China) with an accuracy of 0.0001 g. The results of the stomach content analysis were expressed in terms of the frequency of occurrence (%F), numerical abundance (%N), and wet weight ratio (%W) of each prey organism using the following formulas:
where Ai is the number of S. inermis in whose stomach contents the prey organism was found, N is the total number of S. inermis, Wi is the wet weight of the prey organisms, and W total is the total wet weight. The frequency of occurrence and wet weight ratio were used to analyze the stomach contents of rockfish, and the index of relative importance (IRI) was calculated according to the method described by Pinkas et al. (1971), as follows:
Each IRI was converted to a percentage and expressed as the relative IRI ratio (%IRI), as follows:
To investigate the changes in diet composition by TL, the stomach contents were analyzed in four size groups (≤ 15.0 cm; 15–20.0 cm; 20–25.0 cm; ≥ 25 cm) with 5 cm intervals, considering the smallest and largest individuals. Additionally, to analyze seasonal variations in stomach content, the samples were divided into four seasons: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February). The dominant or rare food items, feeding strategy (specialist or generalist), and niche width of the rockfish were visualized using the graphical method described by Amundsen et al. (1996). This method plots the prey-specific abundance against %F and is calculated using the following formula:
where Pi represents the prey-specific abundance of prey i, Si denotes the weight of prey i in the stomach contents, and Sti represents the total weight of prey i in the stomach contents of the individuals that were observed to have consumed it. Amundsen et al. (1996) proposed a method for plotting prey-specific abundance against prey frequency, which has been widely used to analyze the feeding behavior and strategies of fishes. The higher the prey species or taxon located on the graph, the more likely it was to be interpreted as the dominant prey. If the target species has a narrow diet, it is considered a prey specialist; whereas, if it has a wide diet in the feeding strategy of fish, it is categorized as a prey generalist (Pianka, 1988).
The dietary overlap index according to the trophic guild was calculated using the dietary overlap index (Schoener, 1970) as follows:
where Pxi and Pyi represent the wet weight ratios (%W) of prey organism i in groups x and y, respectively. The overlap index values range from 0 to 1, with values closer to 1 indicating a higher overlap. Following Wallace (1981), values equal to or greater than 0.6 were considered significant.
Results
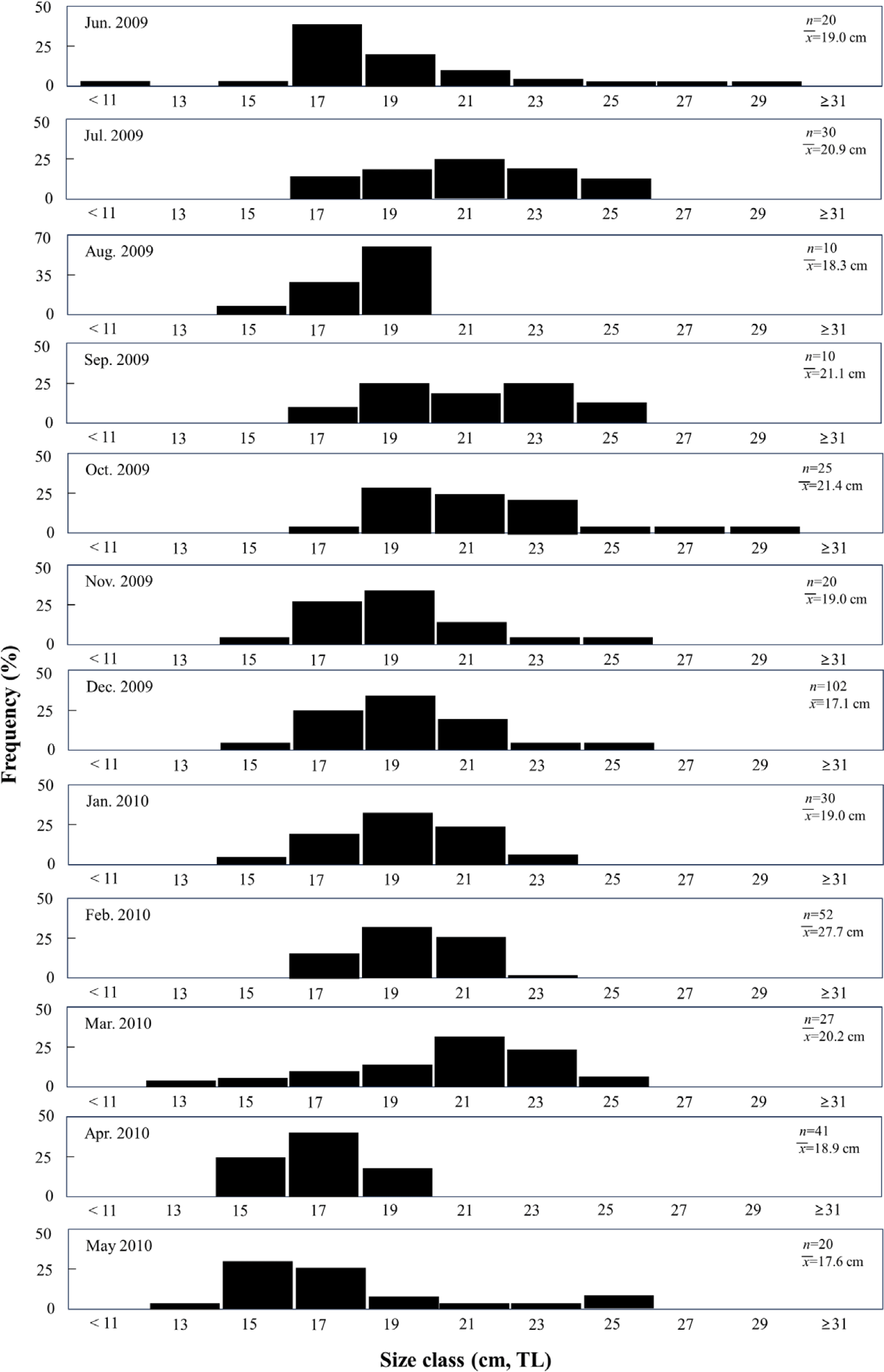
A total of 387 individuals were analyzed in this study, with lengths ranging from 11.7–31.7 cm, among which individuals ranging from 15.0–23.0 cm accounted for 90.7% of the individuals. Regarding monthly length composition, the smallest individuals of TL 11.0 cm appeared in June 2009, followed by an increase in the proportion of individuals of TL > 17 cm after June, and a subsequent increase in the proportion of individuals of TL < 17 cm after April (Fig. 2).
Among the S. inermis used in this study, 162 of 387 individuals had empty stomachs, indicating an empty stomach rate of 42%. Analyzing the 225 individuals that did not have empty stomachs indicated that the most important prey for S. inermis were macrurans (shrimp), accounting for 70.9% of %F, 14.2% of %W, and an of IRI ratio 58.2%. Among the shrimp species, the %W of A. japonicus was the lowest at 2.9%, followed by Crangon hakodatei and Alpheus sp. In addition, Brachyura was an important prey, accounting for 35.3% of %F, 23.0% of %W, and 22.4% of the IRI ratio. Among Brachyura, Charybdis bimaculata had the highest %W (13.4%), followed by Charybdis japonicaand Gaetice depressus. Following Brachyura, Pisces (fish) were the next most important prey, accounting for %F (16.6%), %W (52.1%), and %IRI (14.5%). Among fish, Cynoglossidae was the most consumed, with an occurrence frequency of 8.3%, whereas other prey, such as Isopoda, Amphipoda, Polychaeta, Cephalopoda, and mysidacea, were consumed with very low %IRI (Table 1).
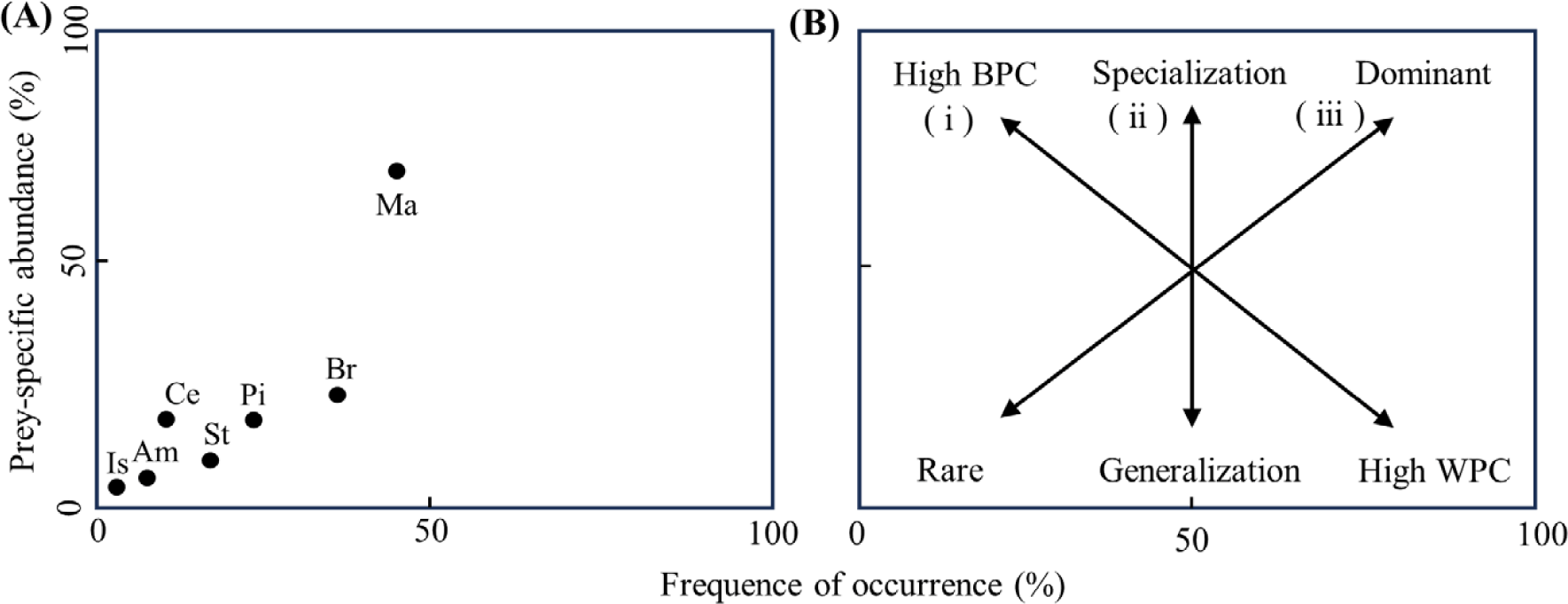
Using a graphical method to analyze the stomach contents of S. inermis (Fig. 3), it was confirmed that the most important prey were benthic animals such as Macrura and brachyura, indicating specialized predation behavior in these groups. Additionally, fish, Stomatopoda, Amphipoda, and Cephalopoda were also consumed, but showed a low occurrence frequency, indicating a narrow dietary breadth for S. inermis.
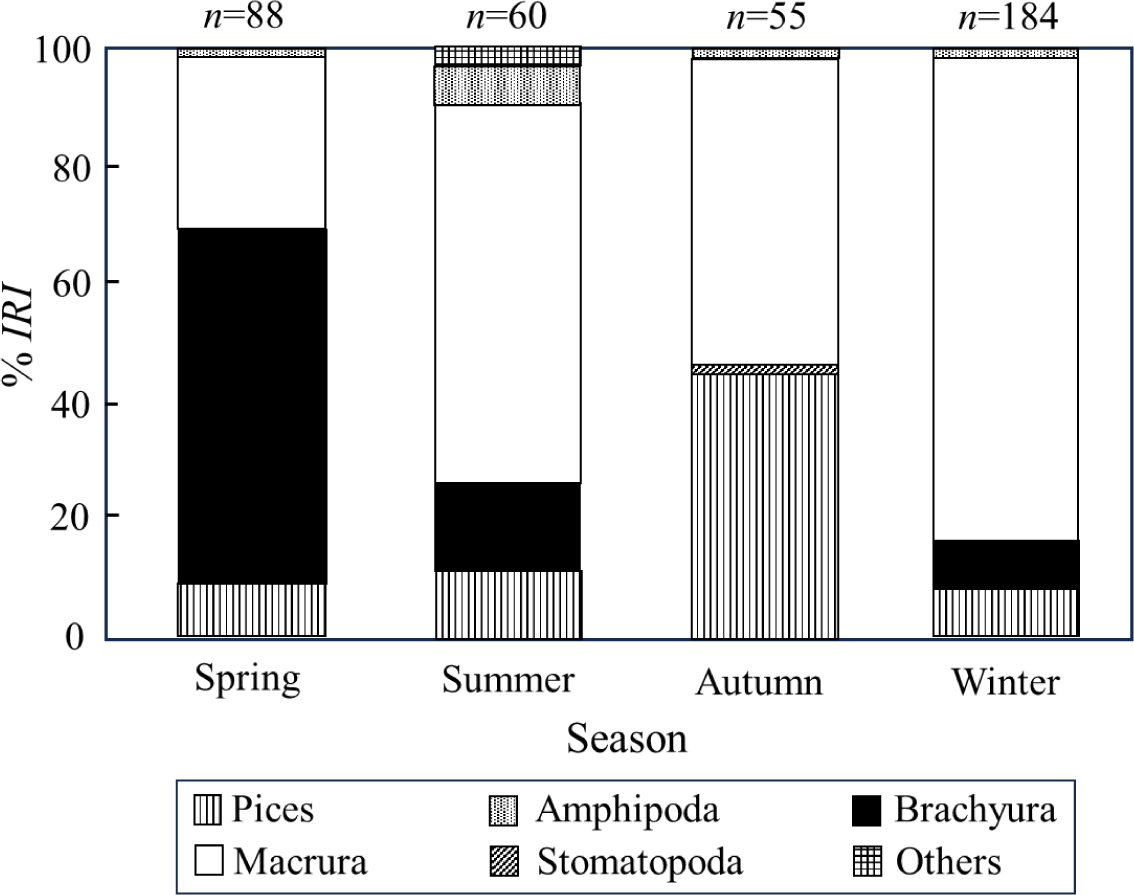
We examined the monthly dietary changes of captured S. inermis, with monthly samples of black rockfish from 10–102 individuals analyzed. To understand seasonal changes in the stomach content composition of S. inermis, we divided the data seasonally, into spring (March to May), summer (June to August), autumn (September to November), and winter (December to February) (Fig. 4). In spring, the proportion of brachyura was the highest at 59.5%, followed by macrura at 30.3%. In summer, macrura dominated (71.4%), followed by brachyura (16.4%), and fish (12.2%). In autumn, although macrura remained predominant at 53.2%, the fish abundance increased significantly to 45.1%, occupying a substantial proportion. In winter, macrura reached the highest proportion (83.8%), followed by fish (8.8%) and brachyura (6.5%). Thus, macrura exhibited the highest rank index ratio throughout the year, with the highest value in winter and the lowest in spring. Crab abundance was highest in spring and lowest in autumn, whereas fish abundance was highest in autumn and lowest in winter.
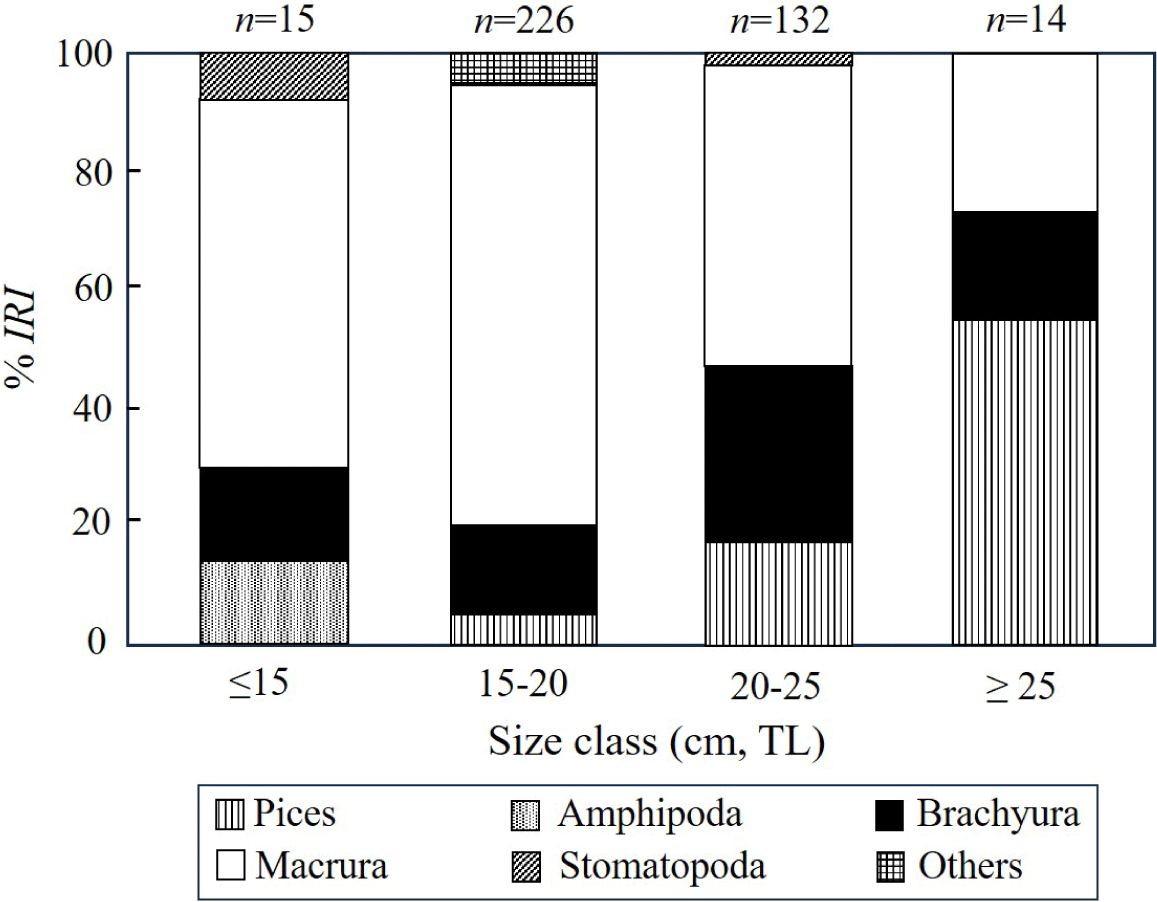
To understand the changes in stomach content composition of S. inermis across size classes, we divided them into four size groups based on TL: ≤ 15.0 cm, 15–20.0 cm, 20–25.0 cm, and ≥ 25.0 cm, and then analyzed the proportions of prey items using rank index ratio (Fig. 5). In the size group below 15 cm, macrura dominated at 62.9%, followed by Brachyura at 15.0%, Amphipoda at 14.1%, and Stomatopoda at 7.9%. In the 15–20 cm group, macrura constituted the highest proportion (75.0%), followed by brachyura (14.7%) and fish (4.8%), showing an increasing trend. In the 21–25 cm group, macrura remained dominant (51.1%), followed by brachyura (28.7%), and fish (17.7%). In the ≥ 25 cm group, fish constituted the highest proportion (54.5%), followed by macrura (26.9%) and brachyura (18.5%). Overall, macrura and brachyura dominated the diet of S. inermis across all size groups, with macrura showing a decreasing trend in the %IRI after reaching its peak at 15–25 cm. Additionally, fish began to appear prominently in the diet from a size of 25 cm, increasing in proportion with increasing size. In conclusion, S. inermis primarily consumed macrura and brachyura; however, the utilization of fish increased as prey increased with size.
Dietary overlap among size classes of S. inermis, assessed using Schoener’s index, revealed a high overlap with values of 0.93 between size classes ≤ 15 cm and 15–20 cm, whereas the lowest overlap was observed with a value of 0.57 between size classes ≤ 15 cm and ≥ 25 cm (Table 2).
| Size class (cm, TL) | ≤ 15.0 | 15–20 | 20–25 |
|---|---|---|---|
| 15–25 | 0.93 | ||
| 20–25 | 0.77 | 0.84 | |
| ≥ 25 | 0.57 | 0.64 | 0.8 |
Discussion
The analysis of the stomach contents of S. inermis collected off the coast of Yeosu revealed an empty stomach rate of 42%. This finding is similar to previous studies on the feeding habits of rockfish, including S. inermis in Jinhae Bay (Kim et al., 2009), and Sebastes schlegelii in Tongyeong (Park et al., 2007), which reported emptiness rate of 20.5% and 38.9%, respectively. These studies collectively indicate that the emptiness rates of rockfish generally range from 20 to 40%. In comparison, other fish species such as Pholis nebulosa at 6.8% (Huh & Kwak, 1997), Trachurus japonicus at 7% (Huh & Cha, 1998), and Pseudopleuronectes yokohamae at 6.5% (Kwak & Huh, 2003) exhibit significantly lower emptiness rates. Conversely, fish species that primarily preyed on larger organisms, such as macrura, brachyura, and fish, showed higher emptiness rates, with Hapalogenys mucronatus at 14.6% (Soh & Kwak, 2005), Chelidonichthys spinosus at 24.1% (Huh et al., 2007), and Scomberomorus niphonius at 30.1% (Lee et al., 2021). This trend suggests that piscivorous species like rockfish, which consume larger prey, tend to have higher emptiness rates due to their specific feeding habits. Understanding these emptiness rates in crucial as they reflect the feeding intensity and frequency, which can be influenced by various environmental factors, including prey availability, water temperature, and seasonal changes (Mihalitsis & Bellwood, 2017). However, the high rate of empty stomachs observed in the analysis of the stomach contents of S. inermis collected from the coastal waters of Yeosu in this study is thought to be due to the rapid digestion rate of carnivorous fish and the higher tendency of active fishing gear, such as gill nets, to have longer immersion times compared to passive fishing gear (Bowman, 1986; Hayward et al., 1989; Vinson & Angradi, 2011).
The detailed stomach content analysis of S. inermis in this study revealed that macrura constituted 58.2% if the diet, brachyura 22.4%, and fish 14.5%, together accounting for 95.1% of the total stomach contents. Although isopods, cephalopods, polychaetes, and mysidacea were also present, they did not form a significant part of the diet. Rockfish in the southern sea of Korea have been found to primary feed on macrura and brachyura (Kim & Kang, 1999). These findings suggest that small rockfish off the coast of Yeosu are bottom-feeding carnivores, beginning their diet with small prey like amphipods and decapods, and progressively shifting to larger prey such as macrura and brachyura as they grow. This dietary shift is indicative of an ontogenetic diet change, a common phenomenon in fish, where the prey size increases with the predator’s size. The predominance of macrura and brachyura in the diet highlights the importance of these crustaceans in the benthic food web and their role in supporting the nutritional needs of growing rockfish.
In numerous studies on Korean coastal fish, smaller individuals have been observed to feed on small prey (e.g., copepods and amphipods), transitioning to larger prey (e.g., macrura, brachyura, amphipods, and fish) as they grow (Kim & Kang, 1999; Baeck & Huh, 2002; Huh et al., 2008, 2011). The dietary habits of S. inermis in Yeosu are primarily composed of macrura and brachyura found in subtidal mudflats, which is consistent with the feeding patterns of Sebastes thompsoni (Park et al., 2007). This study identified specific prey items including penaeid macrura, caridean macrura, and snapping macrura, with brachyura species such as C. bimaculata, Portunus trituberculatus, and Gaetice depressus as the main prey. Similar feeding habits were noted in S. thompsoni near Busan (Huh et al., 2008). Rockfish often consume macrura, with their diet exhibiting seasonal variations based on prey availability (Huh & An, 1997; Yoon et al., 2004). S. inermis inhabiting the coast of Yeosu tended to consume relatively small prey such as brachyura in spring, shifted to primarily consuming macrura in summer, and then reverted to consuming picsces in autumn, before transitioning to smaller prey such as macrura during winter. From these results, it can be inferred that Sebastes spp. primarily feed on zooplankton such as copepods and amphipods when small in size. However, as their feeding areas expand, they consume a variety of prey organisms, including crustaceans such as macrura and brachyura, commonly found in the subtidal mudflat areas. This suggests an adaptive feeding behavior in S. inermis, transitioning from amphipods to crustaceans as they grow.
In terms of changes in the stomach contents of S. inermis with growth, there is a tendency to primarily consume copepods and amphipods of smaller size classes (Akeda et al., 2012; Kim & Kang, 1999; Kim et al., 2009). In the present study, amphipods accounted for 14.1% of the size classes below 15 cm, which is consistent with the observations of previous studies, as these groups were not observed in the larger size classes (Kim & Kang, 1999; Kim et al., 2009). In contrast, macrura showed the highest proportion (75.0%) in size classes below 15–20 cm and 51.1% in the 20–25 cm size class, gradually decreasing in larger size classes, with a significant decrease to 26.9% in size classes above 25 cm. Conversely, the proportion increased gradually from 4.8% in size classes above 15 cm to 54.5% in size classes above 25 cm, showing the highest proportion. Similar trends of increasing fish proportions with growth have been observed in studies of the feeding habits of S. thompsoni and Sebastes pachycephalus (Huh et al., 2008; Park et al., 2007). These changes are interpreted as a transition to a more energy-efficient fish prey with growth, as rockfish develop greater swimming capabilities and require more energy.
Generally, the patterns of dietary transition during fish growth can be classified into three groups (Huh et al., 2011). The first group transitions from amphipods to macrura to brachyura to fish, as exhibited by species such as S. schlegelii (Park et al., 2007), Sebastiscus marmoratus from the Tongyeong coastal waters (Baeck et al., 2011), and Lateolabrax japonicus (Huh & Kwak, 1998b). The second group transitions from amphipods to polychaetes to bivalves to decapods, as exhibited by species such as Acentrogobius pflaumii from the Gwangyang Bay (Huh & Kwak, 1998c) and Cynoglossus joyneri (Baeck & Huh, 2002). The third group transitions from amphipods to macrura to bivalves to polychaetes (Huh & Baeck, 2003). Additionally, there is a form in which similar prey is consumed regardless of growth but transitions to larger prey with growth (Baeck et al., 2010). Therefore, S. inermis inhabiting the coast of Yeosu was found to belong to the first group, initially consuming relatively small crustaceans, such as macrura and brachyura, and transitioning to fish as their main prey with growth. This suggests a strategic adaption in their feeding habits to optimize energy intake and growth efficiency over time. In this study, we observed a gradual shift in the diet of S. inermis towards larger prey, particularly fish, as they increased in size. This transition indicates a strategic response to their changing nutritional requirements and availability of prey items in their habitat. By adapting their feeding behavior to consume larger prey as they grow, S. inermis demonstrates a flexible and adaptive foraging strategy to maximize their survival and growth potential. However, as our study did not include specimens of S. inermis below 10 cm in size, further investigation is warranted to comprehensively understand their feeding ecology and dietary transitions during early stages of development. Future research efforts should focus on analyzing the stomach contents of juvenile specimens and conducting surveys of prey organisms in their habits to gain insights into the feeding ecology of S. inermis across different life stages in the coastal waters of Yeosu.
Conclusion
Of the 387 fish, 162 had empty stomachs. These findings suggest a pattern of dietary transition during the growth of S. inermis, where individuals initially feed on small-sized crustaceans such as macrura and brachyura, gradually shifting towards consuming fish as they mature. This transition likely reflects the growth in swimming capabilities and energy requirements of S. inermis as it develops and opts for more energy efficient prey. However, this study emphasizes the need for further investigations, particularly focusing on younger individuals below 10 cm in size, to fully understand the feeding characteristics and dietary transitions of S. inermis in the Yeosu coastal area. Analyzing the stomach contents from younger specimens and surveying prey organisms in their habitats would provide a more comprehensive understanding of the feeding ecology of S. inermis and contribute to management and conservation efforts for this species and its ecosystem.
