Introduction
The Ethiopian government built vast reservoirs for hydroelectric power generation and agricultural irrigation (Arjoon et al., 2014). Regardless of its intended use, the reservoir’s fishing utilization is increasing; it accounts about 15.65% of the total catch, in importance and has the potential to increase income opportunities for tens of thousands of Ethiopians while also contributing to food security (Teame et al., 2016). However, numerous elements, such as environmental water quality and water hyacinth will eventually have an impact on fisheries and fish community structure in Ethiopian reservoirs (Degaga, 2018; Fetahi, 2019).
Ethiopian inland caught fisheries have increased from 3,500 tons in 1983 to 15,681 tons in 2000 and 18,058 tons in 2010 (Tesfaye & Wolff, 2014). Fish production has increased more than fivefold in the last three decades, according to Tesfaye & Wolff (2014). Fish production from Ethiopian reservoirs is generally low, with a potential of 7,879 tons per year in 2014 (Tesfaye & Wolff, 2014). Several reservoirs, however, have been plagued by issues such as El Nio (Teshome, 2019), Eichhornia crassipes infestation (Firehun et al., 2014), deterioration of water quality (Fetahi, 2019), sedimentation (Wosnie et al., 2020), and destructive fishing methods (Gebru et al., 2019) that have resulted in unsustainable fisheries resources. As a result, the productivity of Ethiopian reservoir fish has dropped (Teshome, 2019).
In Ethiopian water bodies, research was carried out on fish diversity and ecology (i.e., feeding behavior, adaptability, length-weight relationship) (Golubtsov & Darkov, 2008; Golubtsov & Habteselassie, 2010; Wakjira & Getahun, 2017), fish biology (reproduction and fecundity) (Dadebo et al., 2014; Wagaw et al., 2022), and production potential (FAO, 2003; Tesfaye & Wolff, 2014). Unexpectedly, the reservoir fish community and fish production potential, among other characteristics, vary greatly throughout Ethiopia (Tesfaye & Wolff, 2014). As a result, the goal of this study was to explore the value of reservoir fisheries in Ethiopia. The review focuses on the diversity of fish, their production potential, and challenges in Ethiopian reservoir fisheries. The evaluation also includes recommendations for proper reservoir fishery management, conservation of fish diversity and output, and long-term fishery resource exploitation.
The diversity of fish
Ethiopia’s lakes, rivers, and reservoirs are home to a diverse range of ichthyofauna (Damesé, 2012; Golubtsov & Habteselassie, 2010; Wakjira & Getahun, 2017). However, due to a lack of thorough inventories and the need for taxonomic revision for numerous groups, estimates of fish diversity in Ethiopian inland water bodies are still imprecise. Every year, new species are discovered, even in the most studied hydrographic basins (Golubtsov & Darkov, 2008; Wakjira & Getahun, 2017). According to Damesé (2012) Ethiopia’s freshwater fish diversity includes 12 orders, 31 families, and 75 genera, with a total of roughly 200 fish species, including 194 native fish species. Endemic and exotic species are 40 and 11, respectively (Getahun, 2017).
Within geographical locations, the diversity and composition of fish species varies (Wakjira & Getahun, 2017). Recent research on the distribution and structure of fish assemblages in Ethiopian river’s main channel and larger tributaries, lakes, and other aquatic habitats revealed the country’s uneven ichthyofaunal distribution (Getahun, 2007; Vijverberg et al., 2012; Wakjira & Getahun, 2017). This is mainly due to habitat heterogeneity, human activity, intra-community interactions, environmental gradients, amount and character of the tributaries which affects the diversity, distribution and abundance patterns of species and, thus, community structure (Getahun, 2007). The Baro-Akobo basin, for example, has the largest fish diversity, followed by the Omo-Turkana and Blue Nile basins (Getahun, 2017). The Blue Nile and Rift Valley basins, on the other hand, have the highest levels of endemicity (Golubtsov & Darkov, 2008).
Despite the fact that Ethiopia is currently separated from both East African and South Arabian mountains (Tudorancea et al., 1999), two major biogeography units, the Nilo-Sudan and East Coast ichthyofaunal provinces, are in contact with this region (Vijverberg et al., 2012), additionally recognized the Ethiopian Highlands ichthyofaunal province that is entirely within the borders of Ethiopia (Golubtsov & Mina, 2003). As a result, Ethiopia’s freshwater fish fauna is a diverse mix of Nilo-Sudanic, East African highland and endemic fish species (Getahun, 2017). The genera Alestes, Bagrus, Citharinus, Hydrocynus, Hyperopisus, Labeo, Malapterurus, Polypterus, Protopterus and Mormyrus represent Nilo-Sudanic forms of fish species related to West African fish species (Damesé, 2012; Getahun, 2017). They are common in several basins, especially the Baro-Akobo, Omo-Gibe, Tekeze and the Blue Nile, however they are not found in the northern and central Ethiopian Rift Valley lakes (Getahun, 2007; Wakjira & Getahun, 2017).
The Northern Rift Valley lakes (e.g., Lake Hawassa, Ziwai, and Langano), Awash River basin, Highland lakes (e.g., Lakes Haiq and Tana), and tributaries of the Blue Nile basin are home to the highland East African forms (Damesé, 2012; Getahun, 2017; Golubtsov & Mina, 2003). Barbus, Clarias, Garra, Oreochromis, and Varicorinus are their representatives (Damesé, 2012; Getahun, 2017). Fishes from Eastern and Southern Africa, as well as the Arabian Peninsula, are related to them (Golubtsov & Mina, 2003).
The Labeobarbus spp. endemic forms are well represented in the Blue Nile drainage system, which has 23 (57.5%) endemic fish species, far more than any other Ethiopian drainage system (Damesé, 2012; Getahun, 2017; Golubtsov & Mina, 2003). The majority of these species (including large and small Barbus, Nemacheilus abyssinicus, and Garra cf. dembeensis) are found only in the Lake Tana basin (18 species, 45%) (Getahun, 2017). From the total of its 28 fish species, about 75% are endemic (Getahun, 2007; Golubtsov & Darkov, 2008). Beyond the lake Tana basin, a few fish species endemic to Ethiopia are found: some large barbs (e.g., Barbus zaphiri), Varicorhinus beso, Garra species (Garra ignestii, Garra makiensis), Barbus ethiopicus, Danakilia franchettii and Lebias stiassnyae are also included in the accounts of Ethiopian endemic ichthyofauna (Getahun, 2000; Golubtsov & Mina, 2003).
The majority of exotic species have been introduced to water bodies in Central and South East Ethiopia, with the Rift Valley having the most exotic species (Tesfaye & Wolff, 2014). About 11 exotic fish species have been introduced into Ethiopian water bodies, including Tilapia zillii, Tilapia rendalli, Salmo trutta, Oncorhynchus mykiss, Ctenopharyngodon idella, Hypophthalmichthys molitrix, Carassius carassius, Carassius auratus, Gambusia holbrooki, and Esox species (Getahun & Stiassny, 1998; Tedla & Meskel, 1981). C.idella, H. molitrix, G. holbrooki, and Esox species, on the other hand, did not establish breeding populations and so no longer exist in Ethiopian water bodies (Tesfaye & Wolff, 2014).
The fish fauna of the reservoir is primarily dependent on the riverine environment (Gebru et al., 2019; Mequanent et al., 2022; Wakjira & Getahun, 2017). Their dynamics are strikingly comparable to those found in rivers (Gebreselassie et al., 2021; Tesfaye & Wolff, 2014). Nonetheless, dam construction can alter fish faunal structure by interrupting or delaying migrations and other fish movements, as well as influencing the quality, quantity, and accessibility of their habitat, as well as shifts from riverine to reservoir habitats, which are crucial for fish survival (Mequanent et al., 2022; Shewit et al., 2017).
In comparison to other Ethiopian water bodies, qualitative information on the ichthyofaunal diversity in Ethiopian reservoirs is scarce (Gebreselassie et al., 2021; Gebru et al., 2019). Various authors, on the other hand, have reported different fish genera in different tributaries in their river basin (Gebreselassie et al., 2021; Wakjira & Getahun, 2017). Such ichthyofaunal data have been the focus of much research, including studies on environmental influences on spatial and temporal assemblage patterns, fragmentation and endemic species extinction dynamics (Shewit et al., 2017).
The species composition of Ethiopian reservoir fish communities showed large differences. About 48 species of fishes belonging to 7 orders and 16 families have been recorded in Ethiopian reservoirs; of which, 26 species (about 54%) are found in Alwero reservoir. Out of the 48 species and 16 families so far listed, 19 species belongs to Cyprinidae family and contributed about 40% of Ethiopian reservoirs fish fauna. Mochokidae and Alestidae families (4 species each), Clariidae, Cichlidae, and Mormyridae (3 species each) contributed relatively less composition. All fish communities, with the exception of Alwero, Tekeze, Gilgile Gibe 3, and Rib reservoirs, have poor species richness (Table 1). The species composition is relatively high in Alwero Tekeze, Gilgile Gibe 3, and Ribb, with a maximum of 26, 18, 14, and 11 species, respectively. Only 3 species are common in most reservoirs (Table 1). These are Oreochromisniloticus (Nile tilapia), Cyprinuscarpio (common carp), and Clariasgariepinus (African catfish) (Table 1).
The Alwero, Tekeze, Gilgile Gibe 3, and Ribb reservoirs have more fish species diversity because they are located within the floodplains of the Baro Akobo (Gebreselassie et al., 2021; Tesfaye & Wolff, 2014), Tekeze river (Gebru et al., 2019; Goshu et al., 2009), Gibe river basin (Wakjira & Getahun, 2017), and Tana (Blue Nile River) (Mequanent et al., 2022) drainage basins, respectively. The reservoir appears to be stocked with fish from the main river’s annual flooding and inflow waters linked with the reservoirs. The reason behind fish diversity differences among the reservoirs could be habitat variations (Gebreselassie et al., 2021; Mequanent et al., 2022). Baro river is endowed with much water and good habitats that contain a diversity of submergence and emergent aquatic vegetation and phytoplankton (Gebreselassie et al., 2021). This could be contributed to the higher abundance of fish in the river. Furthermore, reservoir morphometric characteristics have an important effect on the composition of fish communities, in particular in interaction with large-scale climatic factors (temperature, rainfall) and productivity (Brucet et al., 2013). This has similarly been confirmed for functional diversity of natural lake and river basin fish assemblages (Gebremedhin et al., 2018; Mequanent et al., 2022).
Fish production potential
Except for some remote and inaccessible water bodies, subsistence fishing is basically carried out in any water body. On the other hand, commercial fisheries are mainly concentrated in the Koka reservoir and Rift Valley lakes of Ziway, Langano, Awassa, Abaya, Chamo and Turkana, and the northern Lake Tana and South Wollo lakes Hayq and Ardibo (Hirpo, 2017). In these water bodies, the major commercially important fish species of the country include O. niloticus, Labeobarbus spp., Lates niloticus, C. gariepinus, Bagrus docmak and C. carpio (Gebreselassie et al., 2021; Gebru et al., 2019; Mequanent et al., 2022).
Despite the fact that most water bodies include a diverse range of fish species, fishermen are continuously on the lookout for the most commercially valuable ones (Gebreselassie et al., 2021; Tesfaye & Wolff, 2014). However, because many of the fishing gears used are nonselective, a portion of the fish catch is frequently discarded owing to market preferences (Gebru et al., 2019; Mequanent et al., 2022). O. niloticus, C. gariepinus, Cyprinus carpio, C. carassius, B. docmak, Labeobarbus intermedius, and Barbus species are the most important species that make up the bulk of commercial catches (LFDP, 1996). However, due to their availability in nearby water bodies, infrastructure (e.g., roads), and transportation facilities, the geographic distribution of these commercially significant fish species varies across the country (Gebreselassie et al., 2021). Tesfaye & Wolff (2014), for example, recorded more than 23 commercially important fish species in Alwero reservoirs, Gambella region (Table 2), although most of these species are not effectively supplied to the big towns, where fish fetches a relatively high price (Gebreselassie et al., 2021; Gebru et al., 2019; Mequanent et al., 2022).
Data from Tesfaye & Wolff (2014).
Data from Tesfaye et al. (2011).
The highest Ethiopian reservoirs’ fish production potential had been predicted to be 7,698 t/year (FAO, 2003). Tesfaye & Wolff (2014), on the other hand, estimated that significant reservoirs (area > 10 km2) have a production potential of 8,059 t/year. Although only a few reservoirs’ fish production potentials have been researched, there are many more reservoirs across the country that has enormous fish production potential. Grand Ethiopian Renaissance Dam (GERD), Megech reservoir, Geray reservoir, Koga reservoir, and other small and large reservoirs in the country are just a few examples. All of these reservoirs have not been thoroughly investigated in terms of their production capacity, and the country’s exact estimated fish production potential has not been released. In Ethiopia, numerous reservoirs are also being built and are expected to be completed in the near future.
Despite the considerable fish production potential in the reservoirs, current catches are still well below the estimated potential yield, accounting for only 35% of the calculated fish production capacity (FAO, 2003; Tesfaye & Wolff, 2014). Tesfaye & Wolff (2014) calculated the fish production potential for Lake Koka to be 1,360 t/year, whereas LFDP (1996) predicted it to be 1,500 t/year. This is much above the current production level of about 625 t/year (Tesfaye & Wolff, 2014). This calculated difference between Tesfaye & Wolff (2014) and LFDP (1996) could be due to the difference year they used data and the different model used to calculate. Tekeze (583 t/year) (Teshome, 2019), Tendaho (500 t/year) (Teshome, 2019), Fincha-Amerti (333 t/year) (Teshome, 2019), Alwero (251.07 t/year) (Mengist & Fakana, 2020), and Melka Wakena (109 t/year) (Teshome, 2019) had lower current fish production than annual predicted the fish yield of 1,065.63 t/year, 1,345 t/year, 1,822 t/year, 436 t/year and 480 t/year, respectively (Tesfaye & Wolff, 2014) (Table 3).
Data from Tesfaye & Wolff (2014).
Data from Mengist & Fakana (2020).
Data from Awel et al. (2020).
Data from Mequanent et al. (2022).
Data from Teame et al. (2016).
This low fish yield from reservoirs is the consequence of multiple factors interacting. Because only a few species account for the majority of the commercial catch (Table 2), interannual fluctuations in population numbers or productivity of these fish might quickly result in a reduction in the overall catch. A lack of financial support could be another reason for the limited number of fish caches (like fishing gear and credit facilities).
Challenges in Ethiopian reservoir fisheries
Exotic water hyacinth (E. crassipes) expanded rapidly in Koka and Aba Samuael reservoirs in the late 1990s (Firehun et al., 2014). Due to enhanced nitrogen and phosphorus nutrients in the lake, E. crassipes was able to grow in the Koka reservoir (Getnet et al., 2020). During its peak invasion, E. crassipes had a significant environmental and socioeconomic impact on the lake (Firehun et al., 2014; Yigermal & Assefa, 2019). Important fishing operations, in-lake transportation, and water quality have all been damaged as a result of the weed’s spread (Getnet et al., 2020). Reduced levels of production, reduced species diversity, poor-quality fish, and growing operation costs linked with E. crassipes blockage have an impact on fisheries, leading in lower incomes for fishermen and higher prices for fish consumers (Dersseh et al., 2020; Tewabe et al., 2017). Furthermore, due to deoxygenation, dissolved oxygen concentrations in the water underneath the mats dropped to as low as 0.01 mg/L, resulting in unfavorable habitats for fish survival (Ongore et al., 2018).
Asmare (2017) also noted the negative effect of E. crassipes on fishermen by raising fishing costs and diminishing the amount of fish captured. During the fishing season, this invasive weed destroyed the fishing gear and restricted fishing opportunities (Damtie et al., 2022; Tewabe et al., 2017). According to a study by Kateregga & Sterner (2009), the declining catch ability of fish due to the growing abundance of E. crassipes has at least temporarily slowed the loss of fish stocks. Fish catch rates could drop by 45 percent, according to Bhattacharya et al. (2015), since E. crassipes mats blocked access to fishing sites, delayed access to markets, and increased fishing costs.
Reservoirs serve multiple community needs and, in this light, their resources are often subjected to severe competition between irrigation farming and fisheries (Mequanent et al., 2022). Therefore, in estimating reservoir fisheries production, the effects of water drawdown caused by irrigation should be considered; as intensive use of reservoir waters for farming reduces the size of the aquatic habitat available to fish production. Migratory fish are impacted by the expansion of irrigated agriculture (Gebremedhin et al., 2018; Mequanent & Mingist, 2019). The Ribb Dam and Weir in Gilgel Abay (Mequanent et al., 2022), as well as the Shini and Gelda rivers (Gebremedhin et al., 2017) have restricted the spawning migration routes of the Labeobarbus species by irrigation techniques, making them the greatest examples (Gebremedhin et al., 2018; Mequanent et al., 2022).
The Tendaho reservoirs in the Afar region of Ethiopia have been the subject of studies on the relationship between water variability and fish production (Teshome, 2019), where the reduction in reservoir surface area and depth is primarily caused by the withdrawal of water for irrigation during the dry seasons rather than by external climatic factors. Due to this occurrence, these reservoirs lost a significant portion of their fish yield in years with high evaporation, low rainfall, and excessive irrigation activity. In most parts of the world, increasingly scarce of water is also a danger to inland fish production (Nachtergaele et al., 2011). Because irrigation systems are not operated to sustain or even improve fishery productivity, productive fishing has typically decreased in these systems as a result of ignorance or a lack of focus (Eid & Hoballah, 2014; Gregory et al., 2018).
Monofilament nets have been identified as the most common illegal fishing method in Ethiopian reservoirs (Assefa et al., 2018; Teame et al., 2016; Tewabe et al., 2017). Because of the enormous volume of fish collected, most fishermen chose to utilize this form of net because the amount of fish caught by legally approved nets is smaller than that caught by monofilament nets (Tewabe et al., 2017). In most reservoirs, fishermen also practiced using nets with reduced mesh sizes (Assefa et al., 2018; Gebru et al., 2019). Small meshed nets frequently catch juvenile fish, inflicting irreversible damage to the fishery (Tesfaye & Wolff, 2014). Beach seines have the greatest negative influence on reservoir fisheries by disrupting fish habitat.
Due to weak management and regulatory mechanisms, the reservoirs are primarily exploited by illegal fishing materials (Tewabe et al., 2017). These activities could have caused a decline in the number and type of species (Tesfaye & Wolff, 2014). For example, Tewabe et al. (2017) reported 18 fish species in the Tekeze reservoir, however Teame et al. (2016) and Gebru et al. (2019) recorded 11 and 15 fish species, respectively, in a recent fish survey. This suggested that the reservoir’s current high levels of illegal fishing activities, fishing pressure, and other anthropogenic activities have an impact on fishery operations (Assefa et al., 2018; Teame et al., 2016). As a result, the loss in fish species and abundance in the reservoir could be due to illegal fishing activities.
Fish are easily perishable; they decay quickly when exposed to high temperatures, which promote the activity of bacteria and enzymes in the flesh, resulting in post-harvest fish losses (Getu et al., 2015). Biochemical and microbiological deterioration changes that occur in fish after death are frequently the cause of post-harvest fish losses (Tesfay & Teferi, 2017). Natural defensive systems in a living fish help to prevent rotting (Lieke et al., 2020). When a fish dies, however, its defensive mechanisms are disabled, and enzymatic, oxidative, and microbiological spoilage begin to degrade the quality of the fish (Akintola et al., 2022). As stated by many investigators, small-scale fisheries sector in Ethiopian reservoirs suffers from serious post-harvest loss every year (Asmamaw et al., 2021; Assefa et al., 2018; Tesfay & Teferi, 2017).
Several studies have been undertaken in the Alwero (Asmamaw et al., 2021), Tekeze (Assefa et al., 2018; Tesfay & Teferi, 2017), Amerti, and Fichawa (Teklu, 2015) reservoirs to assess the types and amount of losses and to propose the activities required to reduce post-harvest losses. The research demonstrated a high level of post-harvest loss of fishery products during pre-processing, processing, storage, and transportation. Fish losses are caused by a variety of circumstances, including infrastructure issues such as refrigerator shortages, transportation, quality fishing gears, and power fluctuations (Asmamaw et al., 2021; Assefa et al., 2018).
According to research conducted by Assefa et al. (2018) and Tesfay & Teferi (2017) reveal post-harvest fish losses in the Tekez reservoir. Tesfay & Teferi (2017) reported the overall post-harvest loss from the entire catch (8,334.12 tons) between 2001 and 2007 was 238 tons (2.9%) owing to size and spoilage. A total of 508,410 Ethiopian birr ($25,420.5) thought to have been lost (Tesfay & Teferi, 2017). Between 2015 and 2018, fish losses accounted 108.9 tons out of 6,234.99 tons after harvest which was 1.75% (Assefa et al., 2018). And, the overall monetary loss per capita found to be 102,816 ETB per year (equal to 3,672 USD/year/individual) (Assefa et al., 2018).
Teklu (2015) found that of the total annual 98,784 kg tilapia (O. niloticus and T.zillii) catch, 6,816 kg (6.9%) lost due to post-harvest loss on the Amerti and Fichawa reservoirs, with 2,076 kg of tilapia discarded due to size discrimination, 1,323 kg due to operational loss, 648 kg due to market access, and 2,497 kg due to spoilage. In the same study, out of a total carp species catch of 31,317 kg, 3,539 kg (11.3%) lost owing to post-harvest loss, with 560 kg of C. carpio thrown due to size discrimination, 2,143 kg due to species preference, and 447 kg due to spoiling. According to Asmamaw et al. (2021), the Alwero reservoir has a significant amount of fish post-harvest loss. Each year, 4,370.4 kg of fish are cached in Alwero reservoir, with roughly 972 kg (22%) of the fish being lost (Asmamaw et al., 2021). The anticipated monetary loss per year was found to be 31,104 ETB, which is equal to $622.08 USD.
The fish productivity level of a specific water body is determined by the effectiveness of the fishing gear and materials (Deng, 2020). However, research indicates that reservoirs’ yearly fish production capacity is still much below the water system’s maximum estimated sustainable yield (Assefa et al., 2018; Deng, 2020; Gebru et al., 2019). The traditional fishing techniques and gear that the fishermen use are to blame for the poor level of fish yield. For instance, the Alwero reservoir’s fish markets are dominated by 23 species (Tesfaye & Wolff, 2014) and the reservoir’s potential annual fish production is estimated to be 436 tons; however, the reported annual production of 251.07 tons is still well below the anticipated reservoirs’ maximum sustainable yield because of a lack of fishing gear (Deng, 2020).
The majority of Ethiopia’s reservoirs are located distant from main roadways, and the fish harvested from those reservoirs is transported to markets by traditional means of transportation (Deng, 2020; Gebru et al., 2019). Transport and other essential infrastructure are the most hurdles to the fishery production systems, according to analyses of the systems in Alwero (Deng, 2020) and Tekeze reservoir (Gebru et al., 2019). According to Dorgi & Gala (2016), the Gambella region has limited access to transportation services, therefore the fishermen had to carry their catch on foot to the closest markets. Few of them utilize motorcycles, cars, and donkey carts to take their catch to marketplaces since they have limited access to transportation (Deng, 2020; Gebru et al., 2019).
Surveys of the current constraints to fish production in Ethiopian reservoirs were undertaken by Gebru et al. (2019) and Mequanent et al. (2022). The main challenge facing fish products in the majority of Ethiopian reservoirs is the absence of prospective market areas (lack of permanent fish market places) (Deng, 2020; Gebru et al., 2019; Mequanent et al., 2022). The fishers also mentioned that they had to deal with marketing constraints like low prices at the landing site, bad road access, expensive transportation, a lack of cold storage, and bad power supplies (Deng, 2020; Gebru et al., 2019; Mequanent et al., 2022).
Fish stocks’ abundance and distribution may be directly impacted by climate change, which would have an impact on fisheries (Barange & Perry, 2009; Bates et al., 2008). According to Recha et al. (2017), it is likely to lead to an increase in the variability of environmental conditions, such as temperature, precipitation, and river runoff. This will have an impact on ecosystems, societies, and economies and put more strain on all livelihoods and food supplies, including those in the fisheries sector (Lam et al., 2020; Suuronen & Bartley, 2014). The ability of an ecosystem to adapt to change will play a significant role in how specific fisheries will be impacted by climate change (Gomez-Zavaglia et al., 2020). The local fisheries and fish stocks will almost certainly suffer as a result of this (Teshome, 2019).
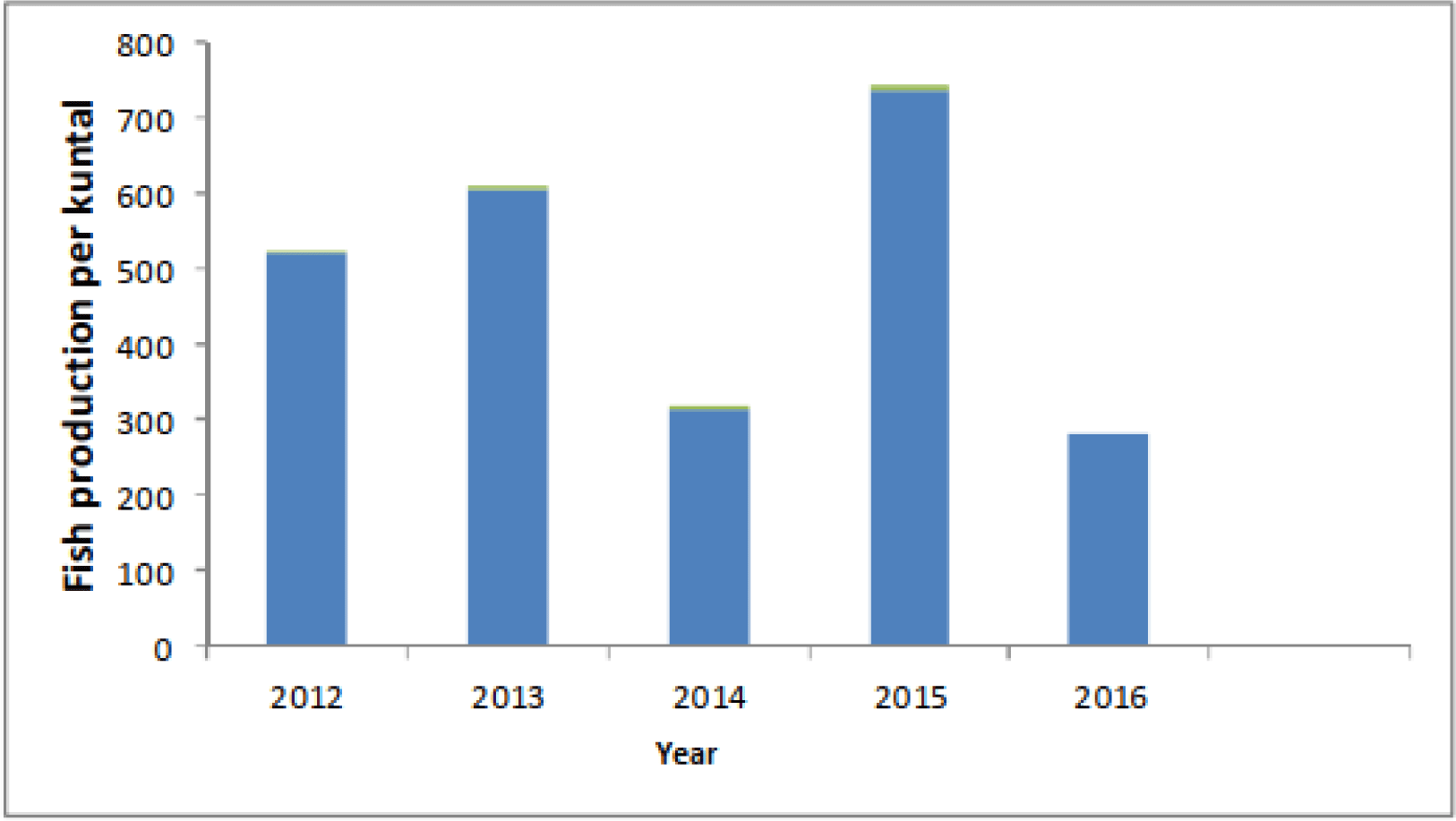
Inland fisheries in tropical regions are especially vulnerable to the effects of climate variability and change (Free et al., 2020). However, a clear indication of climate change effects in the Ethiopian reservoir has been documented by Teshome (2019). Changes in temperature and precipitation are especially prone to affect reservoirs and the rivers that feed them, and extended droughts diminishes the amount of fish habitat available (Gebru et al., 2019; Mequanent et al., 2022; Teshome, 2019). Climate change has caused a severe drought to affect Tendaho reservoir, Ethiopia (Teshome, 2019). Fish stocks are impacted by climate change, which also results in the decline of fishing resources and fish output for human consumption (EFASA, 2011; Lemma & Getahun, 2011; Tewabe, 2015). Higher inland water temperatures decrease the availability of fish stocks by altering the trophic status and water quality of a particular aquatic ecosystem (Teshome, 2019). The vulnerability of fishing households can also increase due to severely climate change impacted agricultural crop. As a result, the only option is to catch any size of fish and the fish population in Tendaho reservoirs has become overexploited (Teshome, 2019). According to Teshome (2019), the Tendaho reservoir’s fish productivity varied between 2004 and 2008 as a result of changes in the reservoir’s water level brought on by climate change (Fig. 1).
Sediment deposition in reservoirs is a serious offsite consequence of soil erosion that threatens the sustainability of dams built for various purposes throughout Ethiopia (Ayele et al., 2021; Haregeweyn et al., 2012). Some reservoirs, such as Koga (Gelagay, 2016) and Koka (Wosnie et al., 2020), have entirely silted up before their planned timeframes. Annual average measured suspended sediment load in Koga reservoir is found to be 62,610.08 tons, and 58,012.87 tons of mean annual sediment yield prediction from Soil and Water Assessment Tool model outputs (Ayele et al., 2021). Also, the capacity of Koka reservoir has been reduced from 1,667 Mm3 in 1959 to 1,186 Mm3 in 1998, according to a recent bathymetric survey (Michael Abebe, 2020). Over the course of 38 years, total capacity has decreased by 481 Mm3, or 28.9% of total storage volume.
Reservoir sedimentation reduces storage capacity and impedes the functionality of intake structures (Ayele et al., 2021; Haregeweyn et al., 2012). This phenomenon in turn can lead to fish-kills caused by decreased oxygen concentrations, algal blooms, and other interrelated consequences. Several studies also show that fish production greatly decreases as the size of the reservoir decreases (Schmutz & Moog, 2018). Silt deposition at the reservoirs is sources of both nitrogen and phosphorus (Wen et al., 2020). Consequently, lead to eutrophication and decline of fish productivity as reservoir surface area and mean depth increase (Schmutz & Moog, 2018). Depth, surface area, nutrient and water chemistry have all been closely related with fish production (Ngatia & Taylor, 2018).
Fisheries management strategies
The fisheries in Ethiopian reservoirs are in grave danger. The community and ecology near the reservoirs are negatively impacted in a number of unanticipated ways by this (Gebru et al., 2019; Mequanent et al., 2022). Therefore, management is necessary to boost the potential for fishing productivity in reservoirs, like that of avoiding using illegal fishing gear for potential danger for recuing fish diversity. Government officials and respected stakeholders should execute management measures include banning damaging gears (such as the use of monofilaments), standardizing and adjusting mesh regulations for different fish species, and implementing some area and seasonal closures.
The management of adaptive ecosystems in response to environmental degradation, pollution control, community empowerment, invasive alien species management, climate change initiatives, and legislation and regulation pertaining to water bodies can all benefit from capacity building and education programs. With a focus on policy adoption of watershed/ecosystem approaches, income generation integration in conservation activities, sharing of responsibilities/benefits among local stakeholders, and institutional strengthening for environmentally and socioeconomically sustainable lake development, both biophysical and socioeconomic aspects of integrated watershed management strategies should be taken into consideration.
Conclusion
The existing fish harvests, which only account for 35% of the estimated fish production capacity, are still far below the expected potential output due to a number of obstructing driving factors. Thus, it has to be boosting particularly reservoir fish harvest using the following actions at least for the coming five years:
-
→ Actively start fishing in the numerous reservoirs that have been neglected yet,
-
→ Link the potential of fisheries and the benefits of the fish farming supply chain,
-
→ Let’s begin building several other reservoirs similar to the GERD and beginning mechanized-based fish harvesting.
-
→ Empowerment of local communities to protect and conserve their resources;
-
→ Efficient methods for managing water resources, such as decision support systems.
