Introduction
In recent years, channel catfish (Ictalurus punctatus) has become an important and highly economical species for aquaculture in the northern region of Vietnam (Hoai et al., 2019; Truong et al., 2020). Diseases in cultured I. punctatus, however, have compromised profitability for farmers. Common diseases observed in I. punctatus include hemorrhage, ulcers, and both internal and external parasitic infections (De la Cruz et al., 2017; Hoai et al., 2019; Wagner et al., 2003). One particularly damaging disease is the “off-white grub” disease, caused by metacercariae of Dollfustrema bagarii (Digenea: Bucephalidae), resulting in substantial economic losses (Kim et al., 2022). Vu et al. (2022) highlighted a staggering 59.7% prevalence of infection in cage cultured channel catfish. Unfortunately, there is currently no effective treatment for this disease in fish.
Ivermectin is widely used to treat parasitic diseases in both humans and terrestrial animals and has shown its effectiveness in treating diseases in aquatic animals as well (Kilmartin et al., 1996; Lorio, 1989; Palmer et al., 1987). Studies have demonstrated its efficacy in treating sea lice on marine fish at three distinct stages through injections (Palmer et al., 1987). Kilmartin et al. (1996) highlighted that salmon is more sensitive to Ivermectin compared to freshwater trout, and both injection and oral administration proved highly effective in treating parasitic diseases. It is worth noting that Ivermectin is a highly toxic drug and can easily kill fish if used in excessive amounts. The signs of Ivermectin poisoning observed in fish include lethargy leading to sinking to the bottom, changes in skin color causing respiratory failure, rapid and erratic swimming, and jumping. However, determining the precise dosage of medication administered via feeding remains a challenge. Therefore, this study focused on evaluating the toxicity of the drug to fish and investigating the optimal dosage for treatment off-white grub disease through injection.
Materials and Methods
The study was approved by Vietnam National University of Agriculture. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Ivermectin concentrations used for injection were determined based on the research findings of Nguyen et al. (2020), when Ivermectin was used to treat an intestinal giant cystic disease caused by Thelohanellus kitauei infecting common carp (Cyprinus carpio). The varying dose concentrations of ivermectin are detailed in Table 1. The fish were closely monitored for a period of 10 days post injection. The effectiveness of the drug was evaluated based on the prevalence and intensity of infection before and after the experiment. The criteria for determining the infection status included assessing the presence versus absence, as well as the viability versus mortality, of D. bagarii larvae inside the metacercarial cysts in the liver, kidney, spleen, and other organs of the channel catfish.
| Injection dose (mg Ivermectin/kg body weight) | Number of experimental fish | Number of recovered fish |
|---|---|---|
| 0 | 8 | 0 |
| 0.10 | 8 | 0 |
| 0.20 | 8 | 3 |
| 0.35 | 8 | 6 |
| 0.55 | 8 | 8 |
| 0.70 | 8 | 8 |
Before commencing the experiments, we conducted examinations on 3 healthy and 10 infected fish to confirm their infection status and quantify the metacercaria load. The healthy fish were found to be free from metacercariae, while the infected fish exhibited a metacercariae density of 3.37 ± 0.99 metacercariae per gram of liver.
We evenly distributed forty-eight infected fish into six distinct experimental tanks, each containing 500 liters of well water. Based on the lethal dose (LD50) and effective dose (ED50) findings, we determined the range of Ivermectin concentrations used in the injection dose, as outlined in Table 2, to find out the suitable therapeutic dose. Three days after the injection, we examined four fish per tank to assess the intensity of infection in various internal organs and observe any changes in the presence of off-white grub on the surface. On the sixth day post-injection, we examined all the remaining fish.
LD50 values were determined using probit analysis in STATA/IC 12 (StataCorp, College Station, TX, USA). We conducted an analysis on infection intensity, measured as the number of metacercariae per gram of liver. The metacercariae counts were log-transformed, and we used linear regression with the dose as a continuous variable. Differences with p-values below 0.05 were considered significant.
Results
Throughout the trial period, the temperature fluctuated between 28°C to 35°C, with an average of 32.67°C. Dissolved oxygen levels varied from 3.5 to 7.5 mg/L, with an average concentration of 5.5 mg/L. The pH values were observed within the range of 6.8 to 7.7, with an average pH of 7.3. Additionally, NH3+ levels ranged from 0 to 1 mg/L, averaging at 0.35 mg/L, while NO2− levels ranged from 0 to 0.5 mg/L, with an average concentration of 0.15 mg/L.
Most poisoned fish exhibited immediate symptoms and died within 2–22 hours, typically within 4–10 hours post injection. Specifically, injecting 2.75 mg of Ivermectin per kg of body weight resulted in the mortality of all fish within 24 hours post-injection. Those fish that survived beyond the initial 24 hours period remained alive until the conclusion of the 96-hour experiment. The LD50-24h value was determined to be 0.808 mg/kg body weight, with a confidence interval ranging from 0.583 to 1.118 mg/kg body weight (Table 3).
No abnormal behavior was observed in experimental fish at the onset of testing the effective dose at 0.10 mg of Ivermectin/kg body weight (Table 1). As the ED100 was determined to be 0.55 mg of Ivermectin per kg of body weight, the calculated value for ED50 was to be 0.253 mg/kg body weight.
At the dose of 0.70 mg Ivermectin per kg of body weight, no fish experienced mortality. However, additional toxic effects were observed, including decreased feeding behavior, lethargy, discoloration, and sinking to the bottom within the initial few hours.
The evaluation of the therapeutic dose ranged from 0.20 to 0.70 mg Ivermectin per kg of body weight. Even after administering an injection of 0.20 mg Ivermectin per kg of body weight, live D. bagarii metacercariae were still detected in the fish, with infection intensities of 2.61 ± 0.37 and 1.80 ± 0.78 metacercariae per gram of liver after 3 and 6 days of treatment, respectively.
After three days of treatment with an injection dose of 0.30 mg Ivermectin per kg of body weight, the infection intensity decreased to 1.26 ± 0.26 metacercariae per gram of liver. By sixth day of observation, no D. bagarii metacercariae were found (Fig. 1). Similarly, injections of 0.40, 0.50, 0.60, and 0.70 mg/kg of body weight with Ivermectin produced positive effect (Table 2).
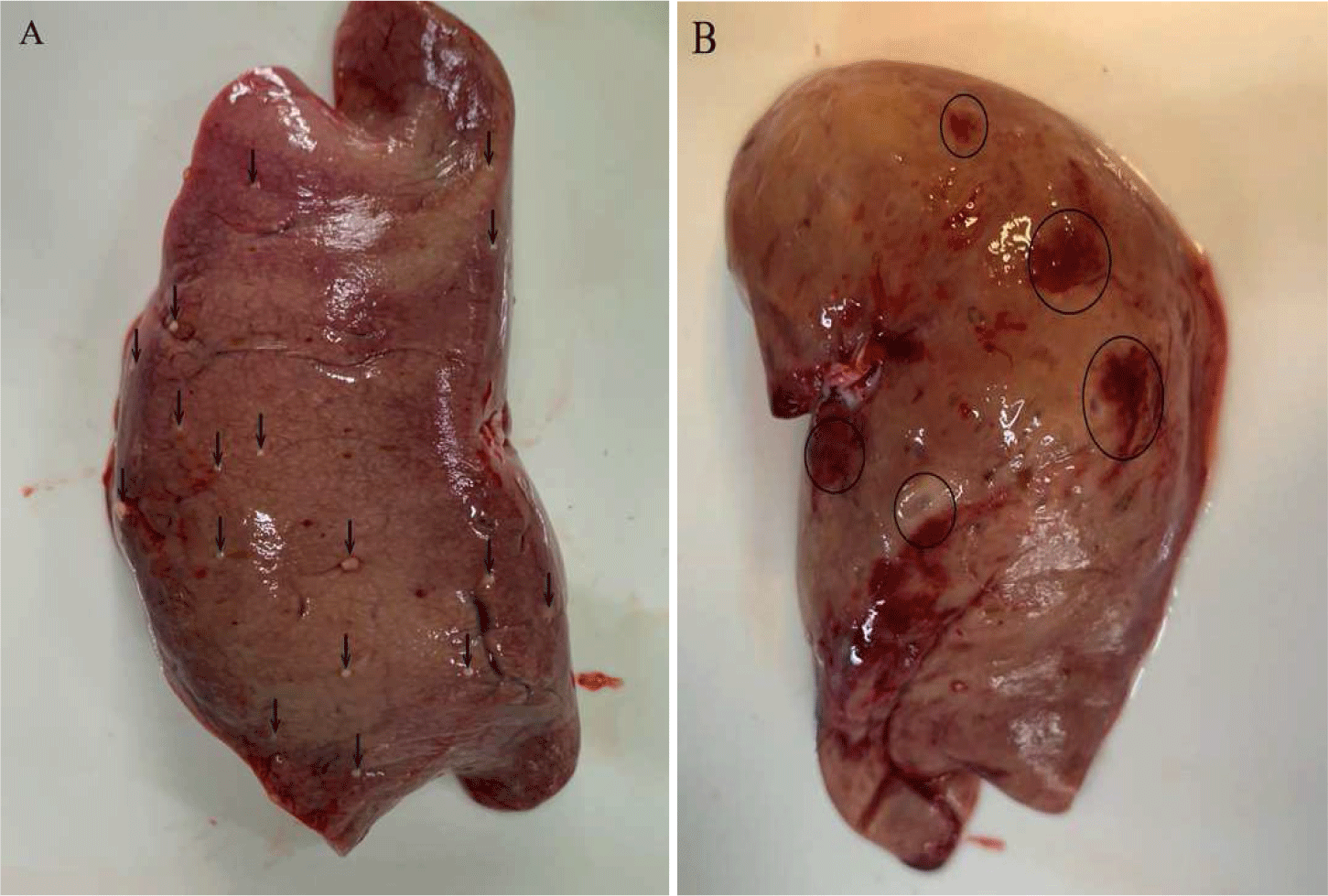
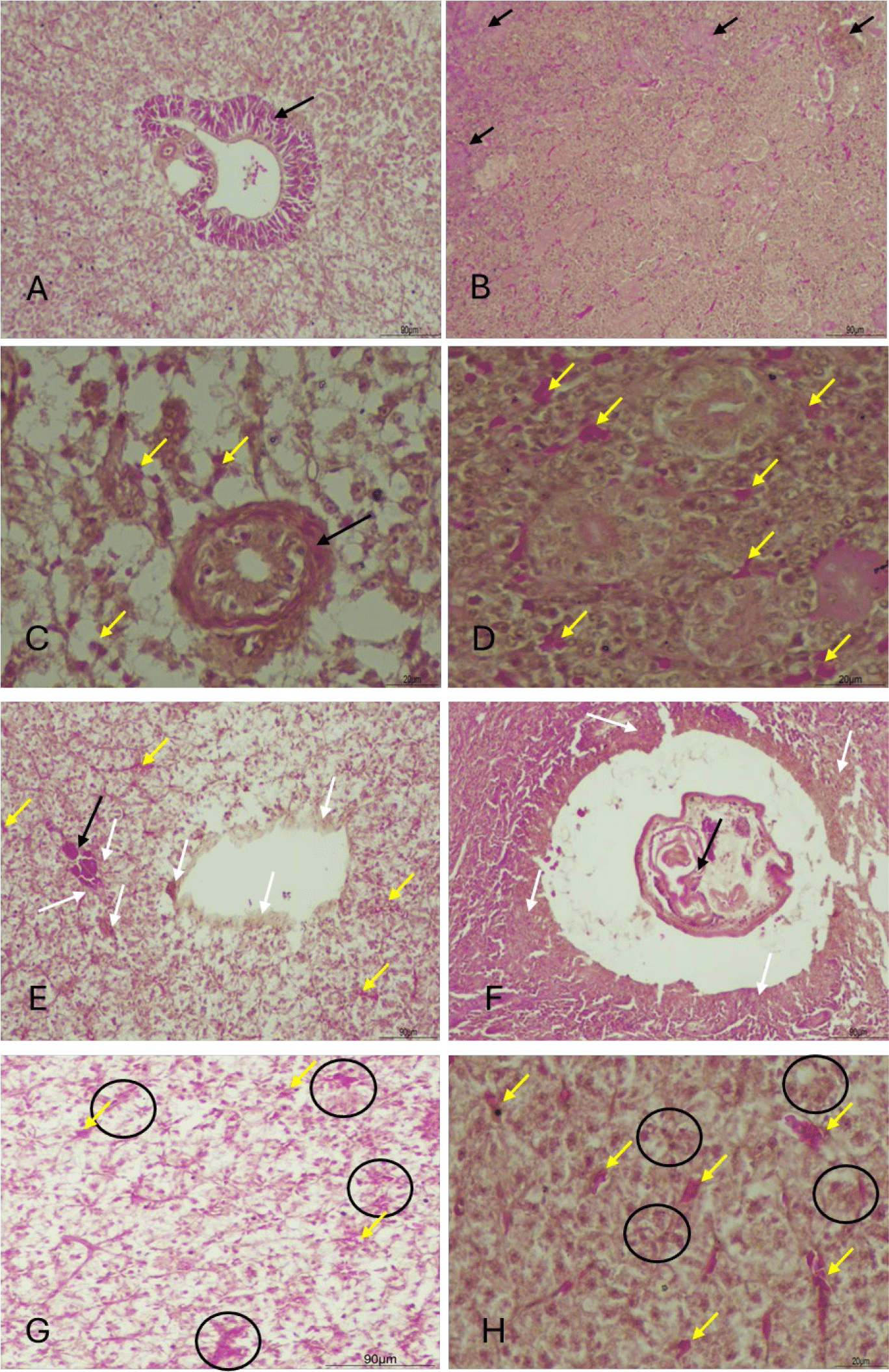
Prior to treatment, metacercariae infesting the liver and kidneys manifested as gross lesions (Fig. 2A–2D). Metacercariae evaded cysts (Fig. 2A and 2B) and infiltrated the tissue, resulting in regions of tissue exhibiting lax structure and hemorrhaging. After six days of treatment, the cysts and metacercariae underwent organ-atrophy, accompanied by the emergence of numerous pigmented macrophages tasked with clearance (Fig. 2E and 2F). The cysts disengaged from liver and kidney, resulting in the formation of concave scar areas (Fig. 2G and 2H).
Discussion
The range of environmental parameters experienced during these trials, as highlighted by Alejandro Buentello et al. (2000), Boyd & Pillai (1985), and Boyd et al. (2002), was suitable for the development of channel catfish.
In aquatic animals, the detection of medical toxicity usually occurs rapidly through observable signs. These signs include restlessness, frantic movement, gulping of air, accumulation of mucus on the body, loss of equilibrium by swimming sideways, and eventual collapse leading to the demise of the fish (Andem et al., 2015). In instances of Ivermectin poisoning due to oral administration in small mammals like mice and rats, symptoms observed encompass vomiting, central nervous system depression, acute respiratory distress, and dilated pupils (Trailović & Varagić, 2007). Throughout our study, fish exhibited poisoning symptoms such as sinking to the bottom, alterations in skin tone, rapid and erratic swimming, and jumping. No additional signs of illness or infection were noded, and notably, all fish swiftly recuperated following the trial. Consequently, based on these observations, it can be reasonably inferred that Ivermectin poisoning served as the primary cause of fish mortality.
Despite numerous studies on Ivermectin’s toxicity to the environment and other organisms, limited data exists regarding the LD50 of Ivermectin injection in channel catfish. Reports on the ecotoxicity of Ivermectin across different aquatic species reveal varying levels: 0.003 ppm for rainbow trout (Oncorhynchus mykiss), 0.048 mg/L for 96 hours in bluegill sunfish (Lepomis macrochirus), 2.5 × 105 mg/L for 48 hours in water flea (Daphnia magna), and over 9.1 mg/L for 72 hours in green algae (Pseudokirchneriella subcapitata) (MSD, 2022; OECD, 2011). It is important to note that LC50 or LD50 doses may differ based on species, drug administration routes, and specific time points (MSD, 2022). Ivermectin is categorized as highly toxic according to the Hazard Classification System - GHS (United Nations, 1992). However, this classification markedly differs concerning terrestrial animals, particularly mammalian species. The LD50 of Ivermectin in rats, mice, and monkeys after oral administration (a single dosage) is approximately 10–50 mg/kg, 25 mg/kg, and 24 mg/kg body weight, respectively. For instance, the LC50-24h by skin contact for rabbits was 406 mg Ivermectin/kg body weight, and LC50-24h for rats was over 660 mg/kg body weight. Ivermectin adversely effects the respiratory system of rats (LC50-24h was 5.11 mg/L of breathing air). In mice, the LD50 via oral administration was found to be 18 mg Ivermectin/kg body weight (Trailović & Varagić, 2007), whereas in beagle dogs, it was 80 mg/kg body weight (Hopper et al., 2002). From this data, it is evident that aquatic animals demonstrate sensitivity to Ivermectin toxicity compared to mammalian species. Additionally, their sizes play a role in their tolerance to Ivermectin toxicity. Therefore, further detailed studies on Ivermectin toxicity in channel catfish are warranted, considering varied sizes and smaller differences between consecutive injection doses.
Beside highly toxic, Ivermectin also caused residuces in food products (Qiao et al., 2023). In 70 days Wang et al. (2020) observed, the concentration of Ivermectin reached a peak in the muscle (0.1921 µg/g) and visceral mass (0.25982 µg/g) of brocarded carp at 7 days after imersing in the water at a concentration of 0.16 µg/L, and then gradually decreasing to 10.179 ng/g in the visceral mass and 3.823 ng/g in the muscle. After administered Ivermectin at the dose level of 0.1 mg/kg bw, the highest total radioactive residue concentrations of 53, 45, and 44 ng/g were obtained on postdose day 1 for hybrid striped bass, largemouth bass, and yellow perch, respectively (Shaikh et al., 2012). Normally, treatment of aquatic animals in large numbers often uses the oral administration. However, there have been no studies to determine residues on channel catfish muscle after injection. Meanwhile, the research of Edwards et al. (1988) in 12 healthy male resulted that after a single dose of 12 mg/kg bw follow oral administration, the maximum plasma concentration reached the highest point at 880 ng/h/mL without any clinical side effects. Those values were lower than the acute oral toxicity in human, at 1,511 mg/kg bw (MSD, 2022). However, the assessment of the effects of long-term absorption needs to be clarified.
Ivermectin demonstrated effective control of “yellow spot” disease caused by Clinostomum marginatum at a dose of 0.22 mg/kg body weight (Lorio, 1989). Similarly, successful treatment of intestinal cysts induced by T. kitauei involved oral administration of 0.6 mg of Invermectin per kg of body weight for three consecutive days (Nguyen et al., 2020). However, studies investigating its applications in channel catfish have been limited. Further research in this subject is essential to enhance the practical ultilization of this medication.
In conclusion, Ivermectin exhibited high toxicity towards channel catfish. An injection dose of 1.406 mg per kg of body weight resulted in a 50% mortality rate among 180–200 g channel catfish within 24 hours. Effective treatment for the off-white grub disease caused by metacercariae of D. bagarii required a dose of 0.2525 mg/kg body weight. Doses ranging from 0.3 to 0.7 mg/kg body weight of Ivermectin successfully treated diseases caused by D. bagarii metacercariae in channel catfish internal organs. Notably, Ivermectin emerged as a valuable treatment for the off white grub disease caused by D. bagarii metacercariae in channel catfish. For practical application in treating large numbers of fish in cages, more research on oral 182 administration is needed.








