Introduction
Globally, there are approximately 2,000 species of marine-derived seaweeds (Periaswamy Sivagnanam et al., 2015); these macroalgae are classified as blue-green, brown, green, and red algae (Widyaswari et al., 2021). About 900 seaweed species have been recorded in Korea, including 123, 193, and 592 green, brown, and red seaweed species, respectively (Kim et al., 2013). Some of these seaweeds have been used extensively as food and medicine (Mabeau & Fleurence, 1993). Seaweeds possess a diversity of components, including proteins, lipids, and fatty acids (Pirian et al., 2018). Apart from these major components, seaweeds produce various secondary metabolites, including polyphenols, peptides, sterols, and a range of other bioactive substances (Wang et al., 2018). In particular, seaweeds from Korea are recognized to have various biological activities (Ryu et al., 2023). However, there is limited information available about the skin-whitening effects of Korean seaweed extracts (Park et al., 2021).
Melanin is a pigment that plays a role in skin, hair, and eye color and provides protection against ultraviolet (UV) radiation’s harmful effects (Costin & Hearing, 2007). Melanocytes, found in the basal layer of the epidermis, are the cells responsible for melanin synthesis (Fitzpatrick & Breathnach, 1963). Melanocytes synthesize numerous secretory melanosomes containing melanin (Kondo & Hearing, 2011). The mature melanosomes in the melanocytic dendrites are subsequently transferred to the adjacent keratinocytes (Beaumont et al., 2011). Therefore, the skin’s final tone is influenced by the amount and type of melanin pigment transferred to the keratinocytes (Wu et al., 2018).
Under standard conditions, melanin synthesis is vital for the protection of deoxyribonucleic acid (DNA) and the skin against UV radiation damage (Ko & Lee, 2021). However, the overproduction of melanin can contribute to the development of skin conditions, such as melasma, freckles, age spots, hyperpigmentation, and lentigo (Rao et al., 2013). These skin conditions can be controlled or inhibited by tyrosinase, a key enzyme in melanin production from tyrosine. Tyrosinase catalyzes the oxidation of L-tyrosine to 3,4-dihydroxyphenylalanine, and then to dopaquinone (Costin & Hearing, 2007). Ingredients known for promoting skin whitening, such as arbutin, azelaic acid, and kojic acid, have been reported to cause side effects, including cytotoxicity, skin cancer, and dermatitis, in long-term use (Huang et al., 2016). Therefore, the development of safe and effective depigmenting agents using Korean seaweeds is of interest. Consequently, this study analyzed the inhibitory effects of tyrosinase in Korean seaweed extracts on the production of melanin.
Materials and Methods
Phloroglucinol, tyrosinase from mushroom (EC 1.14.18.1), L-tyrosine, and Folin–Ciocalteu (FC) reagent were purchased from Merck KGaA (Darmstadt, Germany). The dimethylsulfoxide (DMSO) and sodium carbonate (Na2CO3) were purchased from the Junsei Chemicals (Tokyo, Japan). The kojic acid was purchased from the Tokyo Chemical Industry (TCI, Tokyo, Japan). All reagents and solvents used in this study were of analytical grade.
Twenty-three seaweeds were selected for this study are shown in Table 1. The seaweed samples were obtained from the Marine Biodiversity Institute of Korea (Seocheon, Korea). Each of the provided seaweed samples (brown, green, and red) was stored at −20°C immediately after collection and lyophilized at −40°C with a vacuum freeze dryer (FDT-8650; Operon, Gimpo, Korea). The lyophilized samples were finely ground and extracted three times using an ultrasonicator (WUC-N30H; Daihan Scientific, Seoul, Korea); each extraction was for 1 hr with 70% ethanol. After the extraction step, the samples were concentrated using a Büchi® Rotavapor® R-210 (Merck KGaA) at 50°C. Finally, samples were dissolved in DMSO and stored at −70°C until used.
The total phenolic (TP) contents of brown, green, and red seaweed extracts were determined by the modified FC method (Eom et al., 2011). Phloroglucinol was used as the standard. An aliquot (0.1 mL) of diluted sample was mixed with 0.5 mL of 0.5 M FC reagent in a microcentrifuge tube. After the addition of 0.4 mL of 20% Na2CO3, the mixture stood undisturbed for 3 min. The samples were then incubated at room temperature in the dark for 45 min and subsequently subjected to centrifugation (1,600×g, 8 min). The optical density (OD) of the supernatants was measured at 765 nm using a microplate reader (BioTek Synergy HTX Multi-Mode Reader; Agilent Technologies, Santa Clara, CA, USA). The concentration of TP contents was expressed as mg phloroglucinol equivalent (PGE).
The inhibition of tyrosinase was determined using the method of No et al. (1999), with slight modifications. First, 20 μL of tyrosinase and 10 μL of each seaweed extract (brown, green, and red) with different concentrations (ranging between 1–500 μg/mL) were incubated at 37.5°C for 30 min. Then the assay mixture (170 μL) containing a 10:10:9 ratio of 1 mM L-tyrosine solution, 50 mM potassium phosphate buffer (pH 6.5), and distilled water was added. After incubation at 37°C for 30 min, the OD was measured at 490 nm with the BioTek Synergy HTX Multi-Mode Microplate Reader. The tyrosinase inhibition activities were expressed as half-maximal inhibitory concentration (IC50) values. The percentage inhibition of tyrosinase activity was calculated using the following equation:
The IC50 values were determined using linear regression analysis of the activity observed under the assay conditions. All analyses were performed in triplicate. Kojic acid was used as the positive control.
The previously described reaction conditions were employed to generate a Lineweaver–Burk plot for the seaweed extracts’ enzyme reactions at various concentrations. The identification of inhibition types was characterized by plotting the slope versus the inverse of the substrate concentration.
Results and Discussion
Polyphenols exhibit various biological properties to different extents, including anticancer, antioxidant, and anti-inflammatory effects. The polyphenols of marine algae, called phlorotannins, are synthesized and produced by the polymerization of phloroglucinol (Hakim & Patel, 2020). To evaluate the biological properties of Korean seaweed extracts, their TP contents were determined. The TP contents of the brown seaweed extracts are shown in Table 1. The TP contents of the 12 brown seaweed extracts were between 7.62 and 280.11 mg PGE/g (dry weight); among these, the TP content of Ecklonia cava was the highest at 280.11 mg PGE/g.
The TP contents of the four green seaweed extracts included in the study ranged between 5.24 and 62.37 mg PGE/g (Table 1). The TP content of Cladophora wrightiana var. minor was the highest at 62.37 mg PGE/g, followed by Codium fragile (56.65 mg PGE/g) and Cladophora japonica (5.24 mg PGE/g). The TP content of the Salicornia europaea L. extract was below the limit of detection.
The TP content of the seven red seaweed extracts included in the study ranged between 0.63 and 28.76 mg PGE/g (Table 1). The highest TP content was observed in Grateloupia elliptica (28.76 mg PGE/g), followed by Asparagopsis taxiformis (11.3 mg PGE/g), Chondracanthus tenellus (5.40 mg PGE/g), and Plocamium telfairiae (0.63 mg PGE/g). However, the TP content was below the limit of detection for the Champia parvula, Gracilaria textorii, and Meristotheca papulose extracts. Overall, the brown seaweed extracts exhibited the highest TP contents compared with the green and red seaweed extracts.
To determine the skin-whitening properties of Korean seaweeds in vitro, the tyrosinase inhibition activity of several seaweeds was subjected to comparative analysis. The 70% ethanol extracts of brown, green, and red seaweeds were evaluated for tyrosinase inhibitory effects; the commercial inhibitor kojic acid was included as the control (Table 1). In the 12 brown seaweeds included in the study, E. cava had the highest tyrosinase inhibitory effect with a IC50 of 4.38 μg/mL, followed by Eisenia bicyclis (4.46 μg/mL), Sargassum horneri (6.20 μg/mL), Sargassum yendoi Okamura & Yamada (7.15 μg/mL), and Ishige okamurae (8.97 μg/mL). In comparison, Kim et al. (2005) reported that E. cava exhibited an IC50 of 13.3 μg/mL. Shim & Yoon (2018) reported that the tyrosinase inhibition activity of an E. bicyclis extract had an IC50 of 499.0 ± 6.6 μg/mL. Therefore, the tyrosinase inhibition activity observed in this study was substantially higher than previously reported values.
The four green seaweed extracts screened, namely, C. japonica, C. wrightiana var. minor, C. fragile, and S. europaea L., did not inhibit tyrosinase activity (IC50 > 500 μg/mL). Table 1 shows that the tyrosinase inhibition effect of the green seaweed extracts was relatively lower compared with that of the brown and red seaweed extracts. Therefore, further studies are suggested to identify green seaweeds with IC50 values of over 500 μg/mL.
Table 1 shows that, among red seaweeds, G. elliptica had a higher tyrosinase inhibitory function (IC50 = 281.77 μg/mL) compared with A. taxiformis, C. parvula, C. tenellus, G. textorii, Meristotheca papulosa, and P. telfairiae, which all had lower inhibition against tyrosinase (IC50 > 500 μg/mL). Therefore, there is a need to investigate additional concentrations to identify those with an IC50 of over 500 μg/mL.
Various studies have reported that polyphenolic compounds, which are principal bioactive constituents, demonstrate compelling functional and bioactive attributes. For example, recent studies have identified that seaweed polyphenols have significant benefits, such as anti-viral, anti-bacterial, anti-cancer, anti-diabetes, and anti-inflammatory properties, and are associated with the reduced risk of several diseases (Gómez-Guzmán et al., 2018). Table 1 shows that the TP contents of the seaweed extracts determined in this study were proportionally correlated with their inhibitory effects on tyrosinase. Previous studies have shown that the number and positioning of phenolic hydroxyl groups in polyphenols play a major role in influencing the inhibition of tyrosinase activity (Panzella & Napolitano, 2019). However, the existence of a hydroxyl and electron-donating group within the phenol ring of polyphenols can inhibit tyrosinase activity as a tyrosinase substrate. Therefore, the increased tyrosinase inhibitory effects evident in the brown seaweed extracts, especially E. cava, S. horneri, S. yendoi Okamura & Yamada, and I. okamurae, are associated with their higher polyphenol contents.
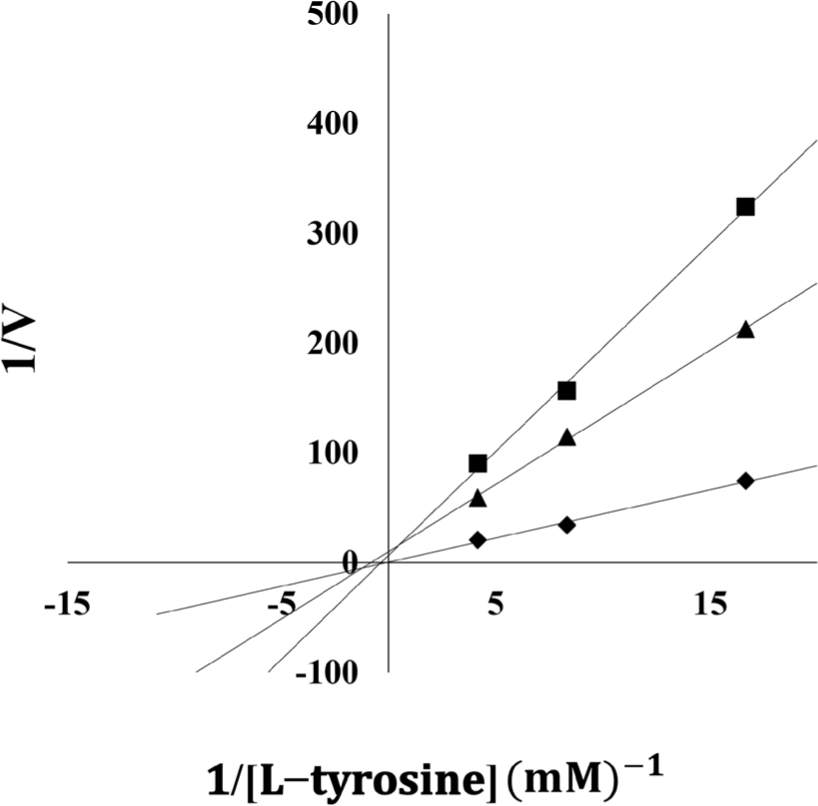
A Lineweaver–Burk plot analysis of the E. cava extract, which demonstrated the strongest tyrosinase inhibition among the seaweeds screened, was carried out to determine the type of inhibition. Fig. 1 shows that the E. cava and E. bicyclis extracts were non-competitive inhibitors; this implies that the extracts’ tyrosinase binds at tyrosinase protein sites other than active sites and does not interact with the substrate at the active site decreasing the enzyme’s efficacy (Blat, 2010). In addition, Lee et al. (2015) have reported that eckol, a polyphenol from E. cava, is a non-competitive tyrosinase inhibitor.
Conclusion
Twenty-three seaweed extracts’ tyrosinase inhibition activity and IC50 values were determined in the present study. Among the brown seaweeds, E. cava extract had the most inhibitory effect on mushroom tyrosinase and was identified as a non-competitive inhibitor in the kinetic study. Furthermore, E. bicyclis (4.46 μg/mL), S. horneri (6.20 μg/mL), S. yendoi Okamura & Yamada (7.15 μg/mL), and I. okamurae (8.97 μg/mL) had higher tyrosinase inhibitory activities compared with the other brown seaweed extracts analyzed. Among the red seaweed extracts, only G. elliptica exhibited a tyrosinase inhibitory effect (IC50 = 281.77 μg/mL). The green seaweed extracts did not exhibit any notable tyrosinase inhibition. The E. cava extract appeared to have a complex composition that warrants further analysis to identify and purify the main components.







