Introduction
Fish resources are globally losing (Tessema et al., 2020). The main causes of the global loss in fish populations, notably in Ethiopia are overfishing of select fish species and environmental deterioration brought on (Mohammed et al., 2016). Despite efforts to maintain a healthy aquatic environment and preserve both fish biodiversity and biomass, fisheries are nevertheless collapsing in some parts of the world (Dadebo et al., 2014). Keystone tool for investigation and management of fisheries include the biometric studies that deliver information on fish species for assessment of their biomass (Hailu, 2014).
Numerous studies have emphasized the significance of establishing biometric relations such as length-weight relations (LWRs) in fish (Engdaw, 2023). The LWRs-derived condition factor, which was developed as a biometric, is significant biometric instrument to develop efficient fisheries management. The condition factor or coefficient of body condition is a useful statistic that demonstrates the health of fish in either their natural environment or aquaculture (Flowra et al., 2012).
Knowledge of fish reproduction biology is essential for managing fish stocks and ensuring their long-term productivity (Hossain et al., 2017). One key biological indicator, the Gonad Somatic Index (GSL), is used to comprehend and evaluate the success of reproduction, which assures the survival of fish species, notably common carp, the study’s subject fish. Shinkafi & Ipinjolu (2012) assert that while estimating reproductive potential, a range of traits, including the age at which sexual maturity occurs, fecundity, the length of the reproductive season, and daily spawning activity must be taken into consideration (Ul-Hassan et al., 2020).
To increase fish production, exotic freshwater fish species have been introduced to a number of man-made and natural water bodies in Ethiopia. The common carp, C. carpio, was introduced to Ethiopia in 1936 for aquaculture (Tegegnie, 2015). It has now been introduced into various reservoirs and other natural water bodies in an effort to fill the void and boost fish productivity. The fish is introduced into the Geray reservoir around 1975. C. carpio is one of the leading contenders for commercial fish production in the reservoir (Mohammed et al., 2016). However, it is dwindling as a result of overfishing and other environmental deterioration. It is essential to manage the reservoir’s fisheries before the total loss of the fish. The goal of this study was, therefore, to assess some biometric and reproductive characteristics of C. carpio to provide information for efficient fisheries management for the reservoir specifically and for the nation generally.
Materials and Methods
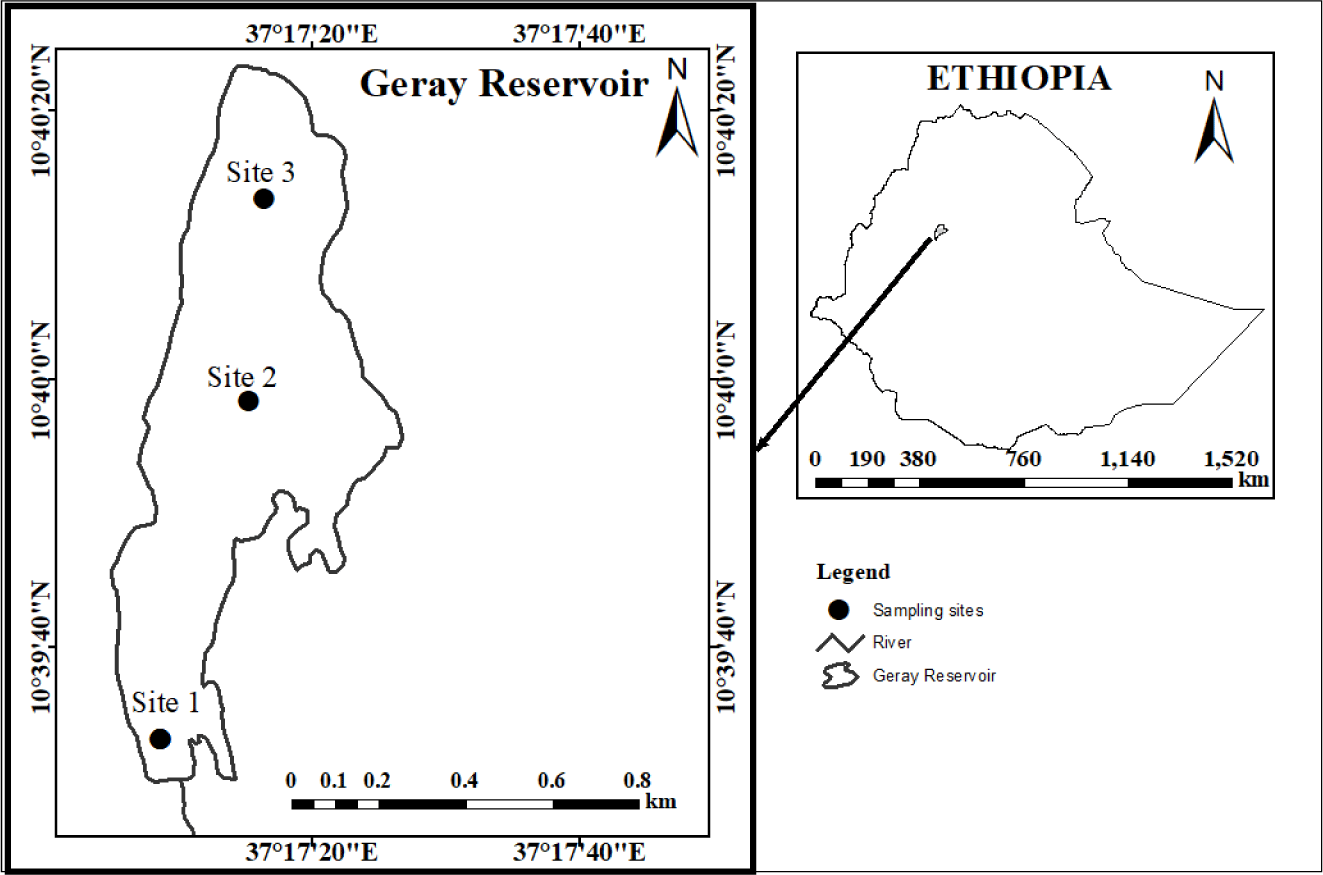
This study was conducted at Geray Reservoir (Ethiopia). Geray irrigation scheme is located 10’ 60’’ latitude and 37’ 26’’ longitude (Fig. 1). It was built in 1972 (Checkol & Alamirew, 2008). It is the largest studied (618 ha) scheme representative of mid-altitude humid climatic conditions in the Amhara Region (Checkol & Alamirew, 2008). The reservoir has a surface area of 10 hectare. The average annual rainfall and temperature in Geray Reservoir, respectively, are 25.75°C and 1,350 mm (Tegegnie, 2015). The reservoir is home to Common Carp (C. carpio), Beso (V. beso), Golden fish (Carassius carassius), and Tilapia (Oreochromis niloticus and Tilapia randelli) (Mohammed et al., 2016).
Fish samples were collected between October and May of 2022 from three landing sites biweekly using cast nets with a mesh size of 6 cm, 8 cm, 10 cm, and 12 cm. It was collected the point closest to the land at the first location, which was a more open area (inlet site). The second fish site was in the area of the marsh that was covered with macrophytes (middle area or pelagic region). Fish from the third location were collected from the main sewage exit (Fig. 1).
Total length (TL in cm) was measured using a normal ruler (48″ model hinges at 24″) and verified against a Vernier caliper to the nearest 0.1 cm. Total weight (TW) was measured using a sensitive digital balance (Model CY510, Citizen, Tokyo, Japan) with a 0.01 g precision.
Each fish specimen was dissected, and the fresh gonads were inspected under a microscope. The sex of the fish species under study was carried out using the approach recommended by Peña-Mendoza et al. (2005) formula:
The LWRs equation W = a Lb was used to estimate the relationship between the weight (g) of the fish and its total length (cm) (Bolarinwa & Popoola, 2013). Using the linear regression of the log- transformed equation: log (W) = loga + blogL. The parameters “a” and “b” were calculated with “a” representing the intercept and “b” the slope of the relationship. The values of “a” and “b” were determined using non-linear regression, and the curve fitting was done using the chi-square test.
The absolute fecundity (AF) for particular females was estimated using a gravimetric approach (Bagenal & Tesch, 1978). The relationship between AF and TL and TW of the fish and their gonad weight (GW) was determined using least squares regression.
The spawning seasons of the fish species under study were detected using the monthly fluctuations of the gonadosomatic index (GSI):
where, Wg is the gonad weight (g) and W is the total weight (g) of the fish.
The wellbeing of C. carpio was determined by using the Fulton condition factor (Bagenal & Tesch, 1978). Fulton condition factor (FCF) was calculated as:
where, TW and TL is the total weight (g) and total length (cm) of the fish, respectively. Good growth condition of the fish was deduced when FCF is greater than 1, while the fish is in poor growth condition when FCF is less than 1.
One-way ANOVA was used to assess the statistical significance of the regression model, length-weight association data and the spatiotemporal variations of fish. T-test was used to determine whether the isometric growth (b = 3) and b predictions for each species were statistically significantly different from one another. χ2-test was used to compare the condition factor between sexes. The coefficient of determination (r2) was used to assess how well a linear regression model predicted outcomes. IBM SPSS Statistics 20 was used to analyses data.
Results
The sex ration analysis showed that C. carpio males (66%) outnumbered females (34%). It fluctuated considerably among the sampling months (t-test; p < 0.05). In the month of October (1:0.25), December (1:0.44), and January (1:0), males significantly outnumbered females (p < 0.05), while in February (1:5) females occurred in significantly higher proportion than males (p <0.05). The overall sex ratio (1:0.52) of males to female of the population when tested statistically showed significant difference (p < 0.05) from the anticipated 1:1 female to male ratio (Table 1).
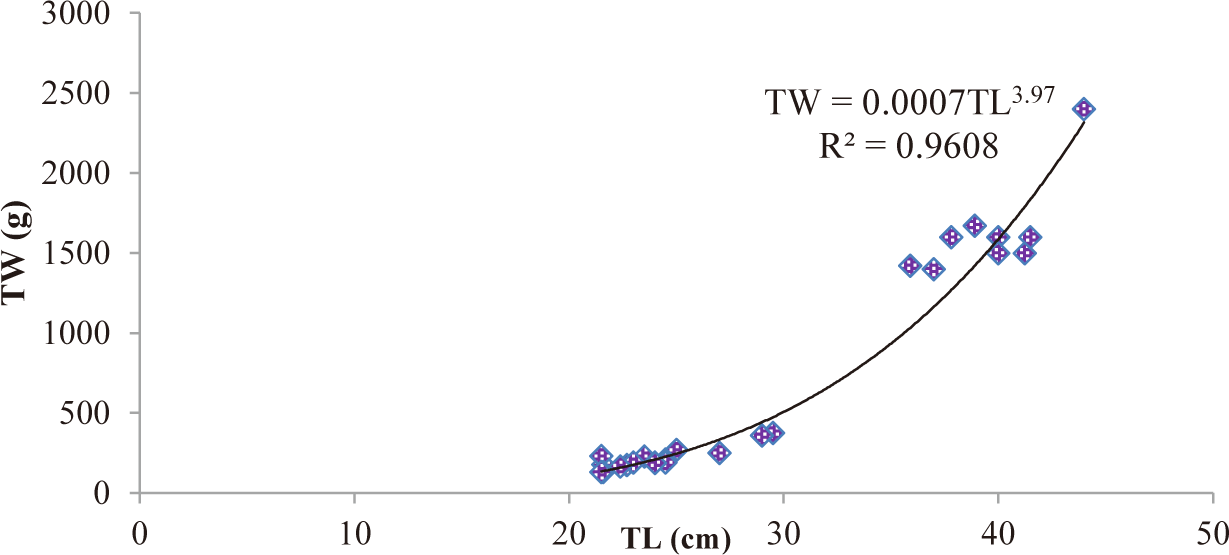
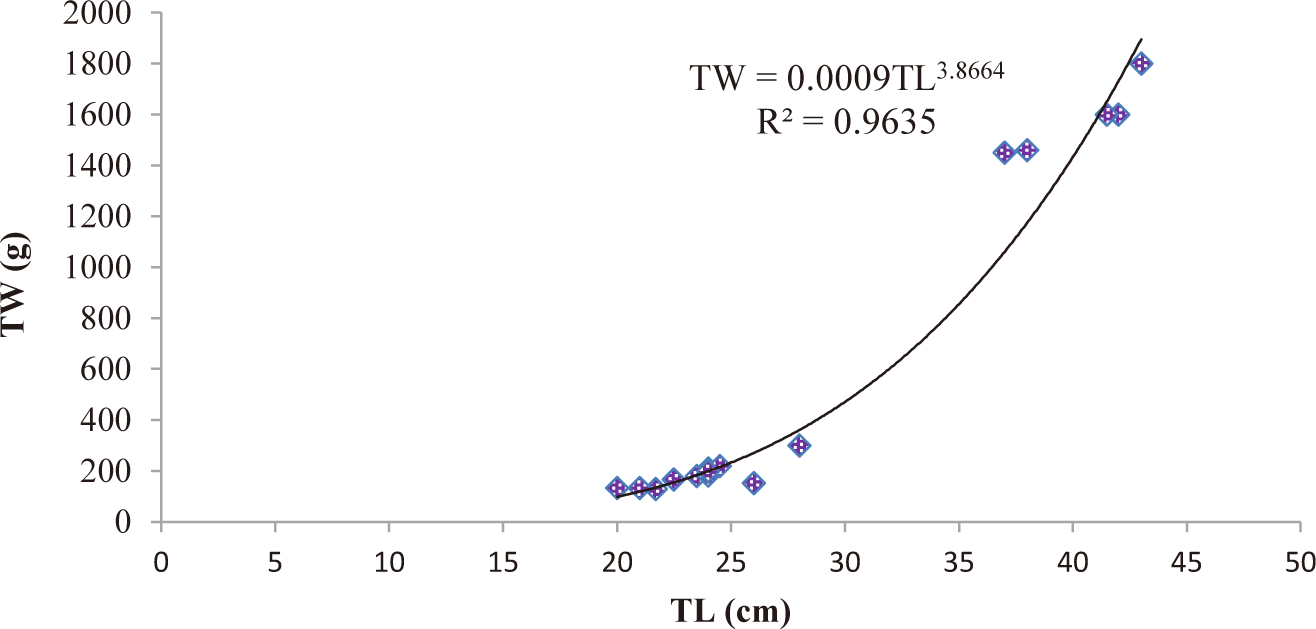
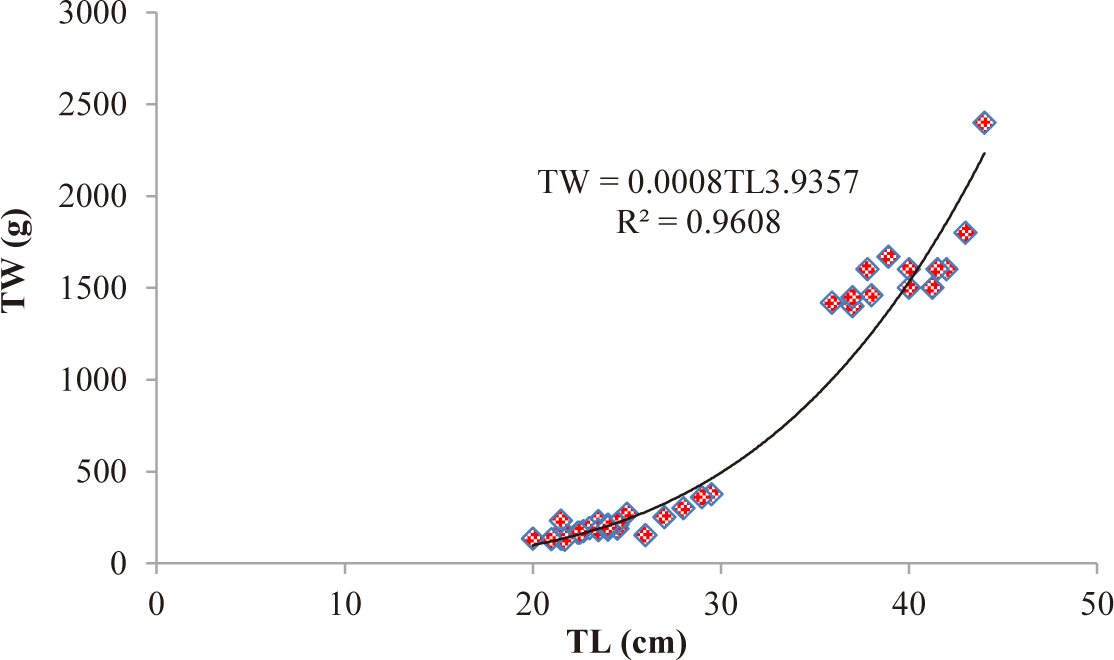
TL and total weight (TW) of C. carpio ranged from 20 cm to 44 cm and from 130–2,400 g, respectively. The relationship between TL and TW of C. carpio was curvilinear. The LWRs of both male (TW = 0.0007TL3.97) (Fig. 2) and female (TW = 0.0009TL3.87) (Fig. 3) C. carpio was strongly correlated, and their r2 value of male (0.9608) and female (0.9635) closed to 1 (Table 2). The “b” values of both male (3.97) and female (3.87) C. carpio were significantly different from 3 (isometric growth), indicating the fish had a positive allometric growth in Geray reservoir (Fig. 4 and Table 2).
| Sex | N | Regression equation | R2 | Sig. | Growth pattern |
|---|---|---|---|---|---|
| Male | 33 | 0.0007L3.97 | 0.9608 | 0.000*** | Positive allometry |
| Female | 17 | 0.0009TL3.87 | 0.9635 | 0.000*** | Positive allometry |
For fecundity analysis, 16 matured female C. carpio with total lengths ranging from 24.9 to 44 cm, weights ranging from 729 to 2,400 g, and gonad weights ranging from 3.2 to 26.6 g were used. This study found that the average AF per fish was 19,920 eggs, ranging from 12,375 to 31,392 eggs (Table 3). Despite being significantly and positively correlated with TL and TW (p < 0.05), AF was not correlated with GW (p > 0.05).
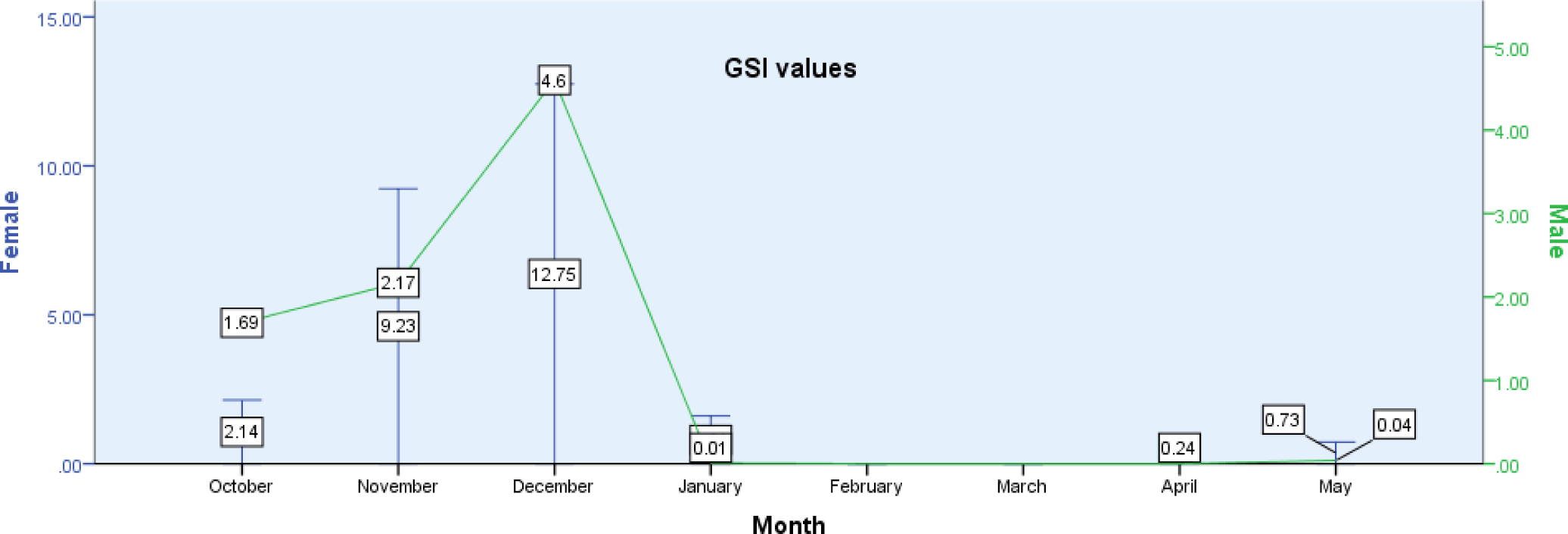
C. carpio had a GSI that varied from 0.01 to 4.6 (male) to 0.24–12.75 (female). The corresponding mean GSI was 1.06375 ± 0.59273 for males and 3.3375 ± 1.72382 for females. The GSI values found in this study fish demonstrated that females had greater GSI values than males in each of stages of gonad maturation. GSI did significantly correlate with total length and total weight of the fish (p < 0.001). GSI revealed monthly variation in both males and females (p <0.05). The maximum GSI for both male (12.75) and female (4.6) C. carpio was observed in December. Relatively high GSI was also seen in October and November. Low GSI was observed in January, April, and May (Fig. 5). It indicated that the breading season of C. carpio in Geray reservoir is extended from December to May with the peaks in December (Fig. 5).
The condition factor (also known as “k-factor”) of C. carpio in the Geray reservoir did not significantly varied between sexes (p > 0.005). However, the mean FCF of females (1.3 ± 0.99) were slightly higher than males (1.3 ± 1.3) (Table 4). The k-factor of both sexes was varied significantly among the sample months (p < 0.05) (Table 4). It ranged from lower values of 1.1 (November and December) to higher values of 1.8 (January) for male and from 1.1 (May) to 1.7 (January) for female. The results revealed that in the sampling months where fish existed, the fish had k-factor greater than 1 (Table 4).
| Month | Cyprinus carpio | |
|---|---|---|
| Male | Female | |
| October | 1.3 | 1.14 |
| November | 1.1 | 1.3 |
| December | 1.1 | 1.6 |
| January | 1.8 | 1.7 |
| February | ND | ND |
| March | ND | ND |
| April | ND | 1.3 |
| May | 1.2 | 1.1 |
| Mean SE | 1.3± 1.3 | 1.36± 0.99 |
Discussion
The study confirmed that C. carpio in the Geray reservoir is dropping because there is high selected overfishing of the fish and unsuitable habitat condition. In the study reservoir, though the total number of fish catch is limited, C. carpio greatly outnumbered females. This sex bias may be caused by sexual segregation during spawning, behavioral differences between the sexes, gear type, and fishing location. The sex difference could be affected by several factors, such as gender-based disparities in mortality, migration patterns, and other behaviors (Anteneh et al., 2023).
The length-weigh relationships (LWRs) provides vital information on the functioning of fish populations (Wagaw et al., 2022) and is an important tool in fish stock assessment to know the growth status and management of the fishes (Ujjania et al., 2012). The LWRs of C. carpio in Geray reservoir is curvilinear, and hence the line fitted to the data that was described by the regression equation. The coefficient (b) values of C. carpio in the study reservoir fell within the range of b values (2–4) for fishes generally, but it is a little higher in tropical fishes (b = 2.5 – 3.5) specifically (Bagenal & Tesch, 1978). Several authors have provided descriptions of the isometric and allometric growth patterns of C. carpio from different bodies of water. The slope of the regression line for both sexes of C. carpio in the current study indicated an allometric (positive) growth pattern of the fish, where the fish’s form and specific gravity do change as it grows in size. The allometric growth of C. carpio in the Geray reservoir shows that the fish is not developing properly, presumably as a result of the inappropriate environmental conditions as well as overfishing. The variation of b can be attributed to a variety of factors, including habitat, seasonal effects, degree of gut fullness, gonad maturity and food availability, sex differences, and environmental toxicology (Sahtout et al., 2017).
Fecundity is among the most essential parameters in studying the reproductive biology of every fish. Knowledge on the fecundity of fish is important to examine the potential of its stocks and actual management of the fishery (Flowra et al., 2012). Fecundity of C. carpio depends on body size and produce between 500,000 and 3 million eggs per spawning (Syed et al., 2020). In the Geray reservoir, the maximum AF of C. carpio is 15 times lower than the minimal number of eggs that the fish is projected to produce. The low AF of C. carpio in the Geray reservoir may be due to the fish’s inappropriate growth as a result of an unsuitable environment. The number of eggs varies greatly, with larger samples producing more eggs than smaller samples (Shinkafi & Ipinjolu, 2012). The smaller sample of fish may be contributed the lower AF in the present study case. Fecundity exhibited a greater correlation with gonadal weight than total length or weight. This suggests that total weight is a stronger predictor of fecundity in this study than total length. The fact that there is a negative correlation between egg size and fecundity indicates that in this species, larger eggs are more prevalent when there are fewer of them. The size of the fish affected its fecundity, so the bigger the fish, the more eggs it produced, maybe because its viscera has more space to retain the eggs.
Fish spawning biology could be studied using a metric called the gonadosomatic index (GSI) (Calagui et al., 2020). In fish, the GSI is a reliable indicator of reproductive activity (Tesfaye et al., 2020). The GSI values found in this study demonstrated that in all six stages of gonad maturation, females exhibited higher GSI values than males. This was correlated with the ovaries’ greater weight while the eggs were present. Females’ TL, TW, and stage of gonad maturation were found to have high relationships with GSI compared to males. Monthly GSI estimations showed that the mature captive C. carpio population becomes ready to spawn twice, from October to December and April to May, with the peak spawning periods occurring in December. Our findings are consistent with the more than one spawning seasons seen in C. carpio in other Ethiopian water bodies such as the Amerti Reservoir (Hailu, 2014), Lake Hayq (Tessema et al., 2020), and Lake Ziway (Tesfaye et al., 2020). This is ultimately because the consistently warm environment. External environmental factors such as rainfall, water surface temperature, pH, hormone release, and turbidity are essential for determining the optimal period for C. carpio reproduction in Ethiopia (Mohammed et al., 2016). In line with the findings of the present study, the peak breeding season was seen in Amerti Reservoir (Hailu, 2014) and Lake Ziway (Tesfaye et al., 2020) during periods of rising water temperature.
Fulton’s condition factor (hereinafter referred to as the “K-factor”) is one of the most often used biometric indices (Batubara et al., 2019). The mean k-factor value of C. carpio in this study for both sexes is larger than 1, implies that the examined fish is in a state of well-being. Fish reproduction cycles, food availability, habitat, and environmental factors are just a few of the variables that affect fish growth conditions (Syed et al., 2020). Comparing individual male and female fish of the same length, but if the female is gravid (full of eggs); comparatively high k-factor is usually observed in females rather than males. Similar results have been reported for other nearby water bodies around the world, such as the Damsa Dam (Mert & Bulut, 2014) and Almus Dam (Karataş et al., 2007) in Turkey, the Foum El-Khanga Dam in Algeria (Sahtout et al., 2017), and the Amerti Reservoir (Hailu, 2014) and Lake Hayq (Tessema et al., 2020) in Ethiopia. During fish spawning in the Geray Reservoir, k-factor results are frequently poor. This could be caused by the lack of food and malnutrition brought on by mouth brooding throughout the reproductive season. During spawning, fish typically reduce their feeding activity and consume their lipid reserves, which results in a decline in condition. The high k-factor values that exist may help to explain why these fish become more aggressive eaters throughout the warm season. In fact, during warmer months when the temperature is optimal an increased k-factor may be associated with more food availability or a higher feeding activity (Asnake & Mingist, 2018).
Conclusion
It is essential to collect area-specific data on the reproductive and biometric aspects such as length-weight relations, condition factor, fecundity, and spawning periods of fish to develop an effective management for fish in their habitats. The biometric and reproductive aspect of C. carpio is the first study on the Geray reservoir which will be used as baseline information to design efficient fisheries management strategies for sustainable utilization of fisheries in the reservoir. The study found that the growth of C. carpio in the reservoir is the allometric growth of fish, indicating the fish are not properly growing as a result of overfishing. Fishermen are strongly urged to avoid catching C. carpio during the breeding season or for a specified period of time until the fish population has recovered.