Introduction
Fish reproductive performance is one of the limiting factors in successful mass larval and juvenile production in an aquaculture setting (Izquierdo et al., 2001). Broodstock nutrition, among other exogenous factors, can significantly affect egg quality and overall seed production. Fish that consume a balanced diet are known to produce high-quality eggs and sperm. It has been studied that the nutritional level of the feed also affects the composition, size, and quantity of eggs (Bobe & Labbé, 2010; Ochokwu et al., 2015). The quality of the egg determines whether a fertilized egg can develop into a healthy embryo and hatch into viable larvae (Bobe & Labbé, 2010). In general, the criteria for measuring the quality of a fertilized egg are determined by assessing data such as fertilization rate, hatching rate, survival activity index (SAI), as well as the shape, size, and biochemical composition of the released egg (Hwang et al., 1999; Kang et al., 2022; Lahnsteiner & Patarnello, 2004; Salze et al., 2005). In addition, biochemical components can significantly influence the initial survival rate of larvae. In particular, the composition of amino and fatty acids in fertilized eggs can serve as a crucial indicator for evaluating egg quality, providing information on the nutritional needs for the initial stages of egg development (Kim et al., 2016; Lanes et al., 2012; Rønnestad et al., 1999).
Fish rely on accumulated substances from their maternal line because the degree of organ development in the larvae stage has limited ability to produce proteins such as hormones on their own (Greenblatt et al., 1989; Lam, 1994). The gene transcript of spawning fish accumulates in mature oocytes during oogenesis and has been studied for its involvement in early embryonic development, directly affecting the quality of fertilized eggs (Reading et al., 2018). The constituents of egg yolk, such as lipovitellin (Lv) and phosvitin, are accumulated by absorbing and decomposing vitellogenin (Vtg), which is produced in the liver of spawning fish (Hara et al., 2016). These constituents make up 80%–90% of the egg mass and contribute to the development of oocytes (Reading & Sullivan, 2011). In addition, growth factors, such as growth hormone (GH), and insulin-like growth factor (IGF), are substances that induce intracellular growth and differentiation, serving as signal transmission factors between cells. It is known that nutritional status affects the expression of these growth factors (Duan, 1997). GH secreted from the anterior pituitary gland can be directly through its receptors or act on the liver to induce an indirect effect through IGF production. The IGF system comprises ligands, binding proteins, and receptors, playing a key role in growth, metabolism, osmotic control, and maturation (Hada et al., 2019; Reinecke, 2010). IGF-1 which are ligand is necessary for the normal development of embryos (Allan et al., 2001). However, their exact roles have not yet been fully identified due to the diversity and complexity of their expressions.
Olive flounder (Paralichthys olivaceus) is a highly cultured, prized, and consumed fish species in Korea and throughout the East Asian region. Specifically in Korea, flounder are predominantly fed a moist pellet (MP) during grow-out and breeding culture, around 90% of the total feed consumption (Kim et al., 2005, 2014; Lee, 2014; Lee et al., 2005; Song, 2011). While MP can induce growth sustenance, a high feed conversion ratio (low feed utilization) triggers pollution in the surrounding environment caused by feed wastage from aquaculture production. This leads to a decrease in dissolved oxygen content and an increase in chemical oxygen requirements and turbidity (Kim et al., 2014; Lee, 2014). One of the viable options as an alternative for MP is an extruded pellet (EP), a nutritionally balanced formulated diet composed of various plant and/or animal nutrient sources. Comparative studies on the effect of EP and MP diets, especially on growth, were demonstrated by previous studies (Kim et al., 2005, 2014; Lee, 2014; Lee et al., 2005; Jeon et al., 2023), however, the effect of these feed types as broodstock diets on the reproductive performance of olive flounder has not been fully elucidated yet. Therefore, this study investigated the effect of feed sources (MP or EP) as broodstock diets on the egg quality and level of growth-related factors of olive flounder eggs spawned in captivity.
Materials and Methods
The current study designated EP and MP as experimental diet types fed to olive flounder broodstocks. EP was a commercially available formulated diet supplied by Daebong LF, Jeju, Korea. Due to company policy, the feed formulation was not made available to the researchers. MP, meanwhile, was a raw fish-based moist pellet composed of trash fish of jack mackerel, chopped, bound, frozen, and thawed before feeding. The experiment was conducted at the institute, Buan, South Korea. A total of 480 olive flounder broodstocks (with a female-to-male ratio of 1:1.3; an average total length of 60.93 ± 0.30 cm and an average total weight of 2,510 ± 45 g) were stocked, with 80 fish in each of six circular concrete tanks. Each tank has dimensions of 2.5 m length × 2.5 m width × 0.9 m deep and a total volume of 17.66 m3. These tanks were designated in a randomized design corresponding to two experimental diets replicated thrice. Fish were reared in tanks with a continuous supply of sand-filtered natural seawater at a flow rate of 80 L per min via a flow-through system and under-shaded natural light (13 L:11 D). The broodstocks were conditioned in this set-up and hand-fed twice daily until satiation with either of the diets for two months before egg collection. Satiation level was determined following the absence of a feeding response. Seawater conditions from the start of feeding until the end of egg collection were monitored and within the normal level for olive flounder aquaculture: temperature (12.3°C–14.8°C), salinity (31.5–32.2 ppt), pH level (7.7–7.8) and dissolved oxygen concentration (6.7–10.8 ppm).
Egg collection commenced in April 2023, two times every week for a total of 9 collection times. Eggs were collected in the morning using a 0.05 mm mesh collection net placed outside the tanks to gather spawned eggs the night before through the effluent stream. For each tank and every spawning event, spawned eggs volume, fertilization rate, egg diameter, oil globule diameter, oil globule volume, hatching rate, malformation rate, and survival activity index (SAI) were recorded. The floating, sinking, and neutral fractions of collected eggs placed in a graduated cylinder were measured volumetrically and summed up to determine the spawned eggs volume. The fertilization rate was calculated by dividing the number of fertilized eggs among 100 randomly selected eggs multiplied by 100. Fertilization was confirmed by the presence of cell division with the aid of a stereoscopic microscope (Olympus, Tokyo, Japan). A photomicrograph of about 100 randomly selected eggs was taken to determine egg diameter (µm) and oil globule diameter (µm), and the oil globule volume was calculated as follows (Lee et al., 1997):
To determine the hatching rate, roughly 100 fertilized eggs were transferred in a 1 L capacity beaker containing 1 L of 1 µm filtered seawater. These beakers were incubated in a 20°C maintained incubator for over 40 h until the eggs hatched into larvae. Half (50%) of the seawater was replaced after 24 h to minimize the effect of water pollution caused by dead eggs. At the end of the hatching period, normally hatched and those with malformation (e.g., deformed vertebrae) were enumerated and the rate was computed by dividing the initial number of eggs multiplied by 100. Subsequently, SAI was determined in normally hatched larvae. Briefly, 50 larvae were transferred to another beaker filled with 1L seawater and incubated at 20°C. Larval deaths were recorded daily until all larvae died. From daily larval mortality data, SAI was calculated based on the Equation (2) (Matsuo et al., 2006),
where N represents the number of larvae at the start, hi represents the accumulated number of deaths by day i, and k denotes the day when all larvae have died.
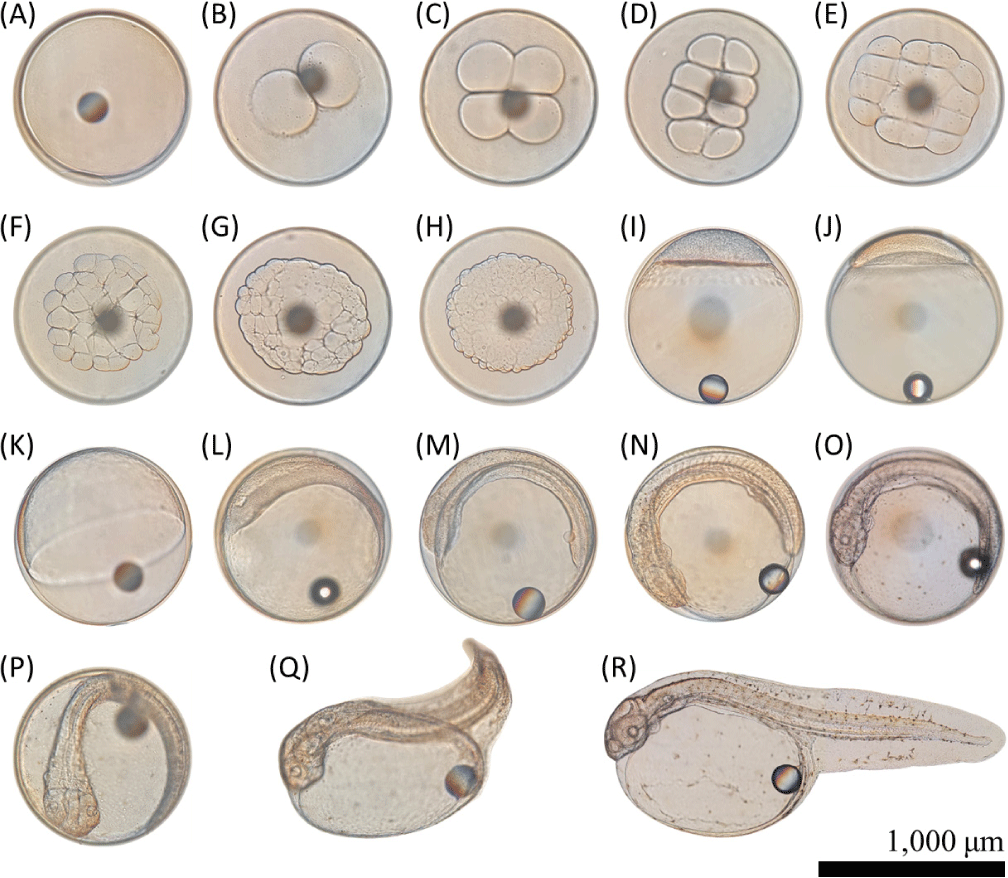
In a separate set-up, samples of fertilized eggs were placed in 2 L glass beakers replicated thrice, incubated at 20°C, and let embryonic development proceed. Time to reach and microphotographs of each of the developmental stages (fertilized egg, 2-cell, 4-cell, 8-cell, 16-cell, 32-cell, 64-cell, 128-cell, morula, blastula, gastrula, embryo formation, Kupffer’s vesicle, eye formation and notochord development, heartbeat stage, before hatching, hatching, and after hatching) were recorded. The development stages were examined using a stereoscopic microscope (Olympus) every 30-min intervals. The time taken to reach a specific developmental stage was recorded when at least 80% of the population had reached that stage.
Samples of each development stage were collected for biochemical composition analysis (except 2-cell, 4-cell, 16-cell, 32-cell, and 64-cell stages). Samples were collected from the beaker using a scoop net and concentrated in 1.5 mL sterile tubes. Tubes were immediately frozen in liquid nitrogen and stored at –80°C until analysis.
Experimental diets and fertilized eggs from each diet were subjected to proximate composition analysis following standard procedures (AOAC, 1995). Briefly, moisture was determined following a constant weighing of samples after drying in a dry oven at 135°C for 3 h. Crude protein was analyzed following the Kjeldahl nitrogen quantification method (N × 6.25) (Metrohm 8-719/806, Herisau, Switzerland). The Soxhlet extraction method was used to analyze crude lipids in a Soxtec extraction system 1046 (Tecator AB, Höganäs, Sweden) using chloroform as solvent. Ash was determined after sample incineration at 550°C for 4 h in a muffle furnace (Daihan Scientific, Gangwon, Korea). Amino acid profiles were determined from hydrolyzed samples (6 N HCl at 130°C for 24 h) and analyzed using high-performance liquid chromatography (Sykam S 433 Amino Acid analyzer, Sykam, Eresing, Germany). Fatty acids were analyzed by Gas chromatography/mass spectrometry (GCMS-QP5050A, Shimadzu, Kyoto, Japan) following Metcalfe & Schmitz (1961).
The level of GH, IGF-1, and Lv in each of the developmental stages were determined using commercially available ELISA kits following manufacturer’s instructions (GH, #C2294012294, Cusabio, Houston, TX, USA; IGF-1, #C2269082255, Cusabio; Vtg, #MBS1603087, Mybiosource, CA, USA). Since Lv has the same affinity for Vtg IgG as it does for Vtg, using the Vtg ELISA kit in spawned eggs is equivalent to detecting Lv (Hiramatsu et al., 1997; Holbech et al., 2001). The 100 mg of stored sample was homogenized with 1 mL of PBS and stored overnight at –20°C. After two freeze-thaw cycles, the sample was centrifuged for 5 min at 5,000×g to collect the supernatant. Optical density was taken at 450 nm using a microplate reader (EZ Read 400, Biochrom, Cambridge, UK). Sample concentrations (pg/mL) were calculated based on the standard curve using curve-fitting software (CurveExpert Basic 2.2.3, Hyams Development).
Levene’s test was conducted after the experiment to confirm homogeneity of variance due to the random selection of fertilized eggs and triplicated analysis. After homogeneity was confirmed, an independent samples T-test was performed using SPSS version 27 (IBM, Armonk, NY, USA). The significance level was set at a 95% confidence interval (p < 0.05). Data are presented as the mean ± standard error of the mean (SEM).
Results
The EP diet-fed olive flounder demonstrated a spawned eggs volume of 14,117 ± 1,039 mL and a fertilization rate of 79.14% ± 1.67%, both of which were higher than those observed in the MP diet. In contrast, the egg diameter was significantly higher at 985.3 ± 4.8 μm in the MP diet (p < 0.05), and the oil globule size was also higher at 179.0 ± 1.0 μm, but the oil globule volume was high at 6.4% ± 0.1% in EP diet. Nevertheless, no significant difference was observed in most parameters (p > 0.05, Table 1).
Meanwhile, the hatching rate was higher at 72.7% ± 3.1% and the malformation rate was lower at 2.14% ± 0.14% in the MP diet (p > 0.05). In difference, SAI was significantly higher at 13.1 ± 0.2 in the EP diet-fed olive flounder (p < 0.05, Table 1).
The approximate composition analysis revealed notable differences between MP and EP diets (Table 2). Moisture content in MP was 34.58% ± 1.18%, significantly higher than that of EP (6.67% ± 0.18%; p < 0.05). Moreover, crude protein content in EP was 56.25% ± 0.59%, significantly higher than MP (42.53% ± 0.83%; p < 0.05). Additionally, EP exhibited a significantly higher crude lipid content of 10.90% ± 0.16%, compared to MP (8.70% ± 0.10%; p < 0.05) and higher crude ash content (13.25% ± 0.05%) than MP (9.53% ± 0.43%; p < 0.05).
The essential amino acids (Arg, Leu, Lys, and Val) and non-essential amino acid (Ala, Asp, Glu, and Gly) showed higher values in both experimental diets (Table 3). Additionally, Ile and Val among the essential amino acids, and Tyr among the non-essential amino acids, were significantly higher in EP compared to the MP (p < 0.05). Gly showed 6.90% ± 0.03% in MP, which was relatively higher than EP (4.81% ± 0.09%; p < 0.05). Moreover, the total of amino acid contains in the diet’s crude protein was significantly higher in MP (p < 0.05).
The fatty acid analysis revealed notable differences in the composition of saturated, monounsaturated, and polyunsaturated fatty acids (PUFA; Table 4). The MP had significantly higher levels of saturated and monounsaturated fatty acids compared to the EP (p < 0.05). PUFA, on the other hand, was significantly higher in the EP (p < 0.05). Specifically, linoleic acid was 2.71% ± 0.01%, γ-linolenic acid was 0.08% ± 0.00%, eicosapentaenoic acid (EPA) was 12.00% ± 0.05%, and docosahexaenoic acid (DHA) was 17.35% ± 0.03% in the EP. These values were significantly higher than those observed in MP, where linoleic acid was 2.50% ± 0.00%, γ-linolenic acid was 0.05% ± 0.00%, EPA was 5.22% ± 0.02%, and DHA was 9.42% ± 0.11%.
The proximate composition of the fertilized eggs from EP diet or MP diet-fed olive flounder broodstocks is shown in Table 5. The moisture content in the EP was 90.8% ± 0.10%, significantly higher than that in the MP (89.0% ± 0.20%). The crude protein content was 7.71% ± 0.14% in the MP, significantly higher than the EP (6.37% ± 0.05%). Additionally, the crude lipid content was 1.13% ± 0.01% in the MP, significantly higher than the EP (0.94% ± 0.01%). The crude ash content was 1.36% ± 0.03% in the MP, significantly higher than the EP (1.17% ± 0.01%; p < 0.05).
| Composition (% as-is basis) | Experimental diet | |
|---|---|---|
| EP | MP | |
| Moisture | 90.8 ± 0.10* | 89.0 ± 0.20 |
| Crude protein | 6.37 ± 0.05 | 7.71 ± 0.14* |
| Crude lipid | 0.94 ± 0.01 | 1.13 ± 0.01* |
| Crude ash | 1.17 ± 0.01 | 1.36 ± 0.03* |
The amino acids profile of fertilized eggs revealed that essential amino acids (Arg, Leu, Lys, and Val) and non-essential amino acids (Ala, Asp, and Glu) showed higher values in both experimental groups (Table 6). Sulfur-containing amino acids, Met and Cys, were significantly higher in EP (p < 0.05).
Based on the result of fatty acid analysis of fertilized eggs (Table 7), EPA and DHA values were found to be 8.03% ± 0.07% and 20.62% ± 0.10 % in the EP, significantly higher than values observed in the MP, where EPA was 3.89% ±0.02% and DHA was 18.46% ± 0.01%. In contrast, linoleic acids and arachidonic acids (ARA) were significantly higher in the MP diet (p < 0.05).
The embryonic development of fertilized eggs from EP or MP-fed olive flounder broodstocks was recorded and compared between the two diets (Table 8 and Fig. 1). There was no time difference observed among each replicated experimental set. The cleavage was initiated within 1 h in both diets. In the EP group, the 4-celled stage was reached at 1 h and 30 min after fertilization, while the MP group achieved this stage 10 min later. Development progressed with the EP group reaching the morula stage 6 h post-fertilization and the MP group reaching the morula stage 30 min later. Subsequently, blastula was observed after 11 h and 30 min in the EP group versus 12 h in the MP group. At 15 h and 30 min, the EP group developed into gastrula while the MP group transformed into gastrula stage 30 min later. Developmental stages further progressed with the EP group faster than the MP group. Eggs hatched into viable larvae at 43 h in EP and 44 h in MP post-fertilization.
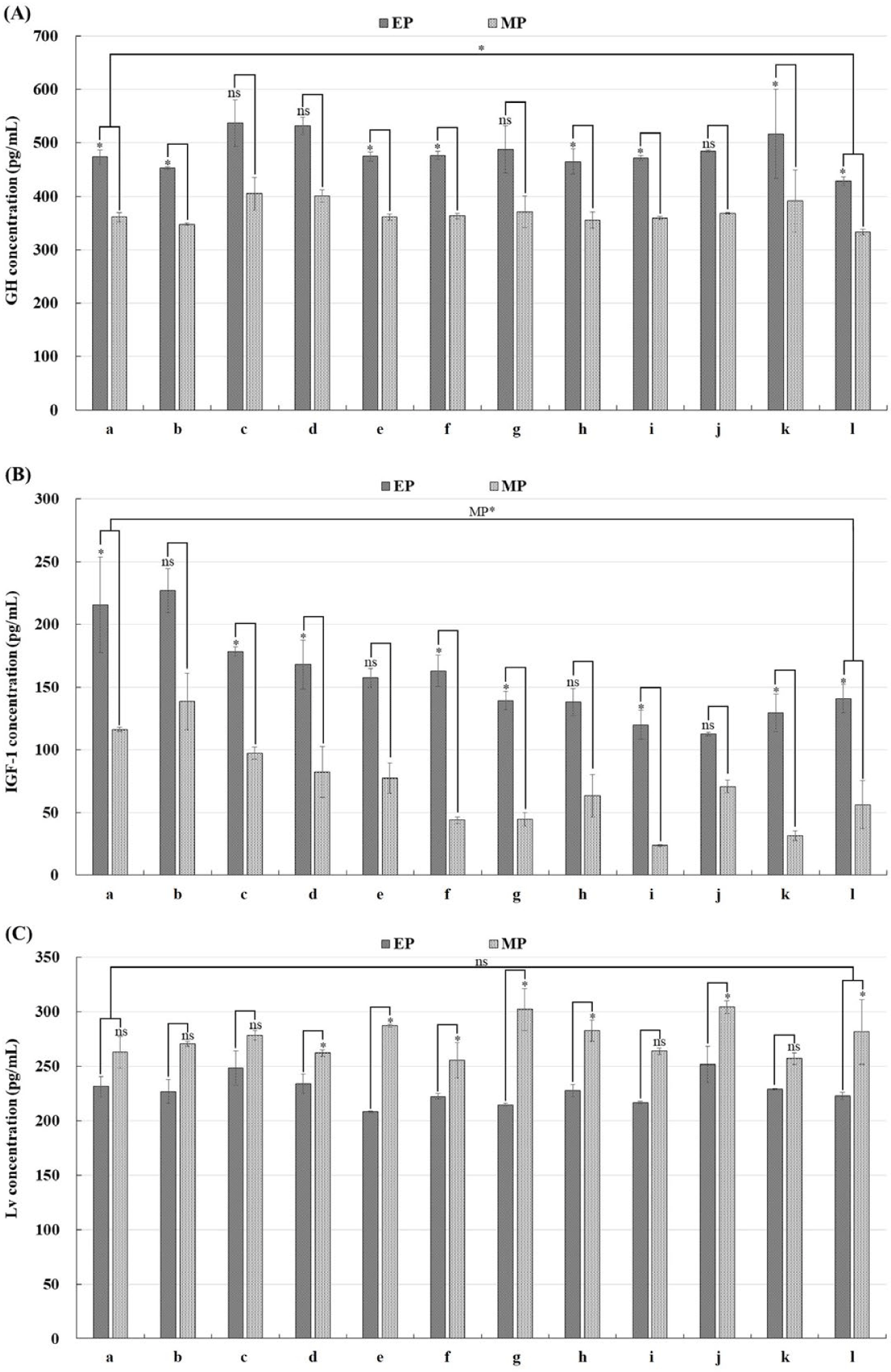
The concentration of GH was significantly higher in the EP group than in the MP group throughout all developmental stages analyzed (except in 128-cell, morula, embryo formation, and heartbeat stage) (p < 0.05; Fig. 2A). IGF-1 concentration was significantly higher in the EP group compared to the MP group (except in 8-cell, blastula, Kupffer’s vesicle, heartbeat stage) (p < 0.05; Fig. 2B). In contrast, the concentration of Lv was found to be non-significantly higher in the MP group than EP group, except in fertilized egg, 8-cell, 128-cells, eye formation and notochord development, and before hatching stage (p > 0.05; Fig. 2C). Notably, there was no significant difference in Lv values immediately after fertilization and before hatching (p > 0.05). However, GH and IGF-1 showed significant differences in values immediately after fertilization and before hatching (IGF-1 showed significant differences only in the MP group) (p < 0.05), and IGF-1 gradually decreased as embryonic development progressed (Fig. 2).
Discussion
Identifying the effect of MP and EP diets on reproductive performance and egg development would be incremental in the continuous production of olive flounder seeds. Based on the result of the current study, egg quality was comparable between the two diets. The egg diameter and oil globule size in the MP group showed higher values compared to the EP group. Despite this, the EP group exhibited better spawned eggs volume, fertilization, and hatching rates, even with smaller egg sizes. SAI, representing the nutritional status, is a crucial indicator for evaluating the activity of hatched larvae. In this study, significant differences were observed in SAI between groups, a higher value was observed in the EP group. These results suggest that the EP diet can be utilized as a broodstock diet without detrimental effects on the quality of spawned eggs. The small variation observed in the study can be influenced by other factors such as age and size of the parent fish, but unlikely by exogenous feed.
It is well-established that the nutrients obtained from the broodstock significantly impact the stable hatching and survival of the larvae (Watanabe, 1993). The proximate composition of the experimental diets indicated that the EP feed was nutritionally superior to the MP feed. However, fertilized eggs have higher crude protein, lipid in the MP. Nevertheless, the variation in the nutritional content of diets and of the spawned eggs did not significantly affect the egg quality parameters of olive flounder. Based on previous studies, Gunasekera et al. (1996) reported that differences in protein levels in the diet did not affect spawned eggs volume, egg diameter, and fertilization rate. In addition, Sarih et al. (2019) demonstrated that varying dietary protein content did not improve spawning quality parameters, yet showed that dietary taurine or histidine can enhance egg quality in greater amberjack (Seriola dumerili). In the current study, although EP was nutritionally superior to MP, there might be other unknown factors that have affected its assimilation by the fish that resulted in less transfer of nutrients from the diets into the eggs. Additionally, elucidation of the specific effect of amino acids and their balance in the diets would further explain the nutrient usage of broodstocks for better egg quality. These warrant further investigations.
In this study, it was observed that Met and Cys concentrations in the fertilized eggs from EP-fed broodstocks were significantly higher than those shown in the eggs from MP-fed broodstocks. Met is an essential amino acid and is made into S-adenosylmethionine (SAM) in combination with adenosine triphosphate (Wu, 2009). SAM delivers methyl groups to DNA, protein, or lipid methylation, of which DNA methylation plays a major role in embryonic development (Kim et al., 2009). In addition, it is converted to Cys, and it is involved in the synthesis of glutathione and taurine (Ekmay et al., 2016). Cys is a sulfur amino acid that directly transports sulfur, and H2S, the product of cysteine, plays a signaling role in neutral metabolism (Li et al., 2009). In poultry, injection of Met and Cys into broiler chicken (Gallus gallus domesticus) embryos enhanced embryonic development and IGF-1 gene expression (Elwan et al., 2019; Mohammadrezaei et al., 2015). In the study by Fontagné-Dicharry et al. (2017), fry of rainbow trout (Oncorhynchus mykiss) that received Met from the parent in embryogenesis, and supplemental Met in their feed showed improved growth and upregulation of metabolism-related genes. Thus, these results may be, to some extent, linked to the elevated concentrations of GH and IGF-I in the fertilized eggs from EP-fed broodstocks, which needs future studies to investigate underlying mechanisms on a role of sulfur-containing amino acids for early development of the fertilized eggs.
In terms of fatty acid content, the EPA and DHA contents in the EP feed used in this experiment were higher than those in the MP feed, while ARA exhibited a higher content in the MP feed. The composition of fertilized eggs produced after feeding each type of feed revealed higher levels of EPA and DHA in the fertilized eggs from the EP group than in those from the MP group, while ARA was higher in the MP experimental group. In particular, the fatty acid in the feed that fed broodstock is directly involved in egg composition (Tocher, 2010). Lipids play an important role in the supply and absorption of fat-soluble vitamins, cell membrane formation, hormone and bile secretion, accumulation of the energy in the body, as well as on egg and larval development (Izquierdo et al., 2001; Tocher, 2010). PUFA such as EPA, DHA, and ARA, which account for 30%–35% of the fatty acid in fish, constitute major organic components in fish, playing significant roles as sources of metabolic energy for growth, reproduction, and movement, including migration (Tocher, 2003; Watson & de Meester, 2014). A high DHA content in the feed provided to broodstock significantly increases the survival of hatching larvae, and a higher content of fatty acids containing DHA and EPA in fertilized eggs corresponds to better egg quality (Izquierdo et al., 2001). However, in the study of Furuita et al. (2003), when mixed feed containing high levels of ARA was fed to olive flounder, it resulted in a rapid end to the spawning season, a low amount of spawning, a low hatching rate, and all the larvae died within 3 d of hatching. Additionally, in a separate study by Furuita et al. (2007), feeding a diet with high levels of ARA to Japanese eel (Anguilla japonica) negatively affected embryogenesis. These studies suggest that excessive levels of fatty acid content lead to a lack of egg yolk and a decrease in survival after hatching. Therefore, the relatively low hatching rate observed in the MP group can be attributed to the low EPA and DHA, and high ARA content compared to the EP group.
During oogenesis, maternal-derived components such as gene transcripts, nutrients, and hormones are absorbed by the developing eggs and stored in the egg yolk, which will provide nutrition during embryogenesis and post-hatching until exogenous feeding commences (Lubzens et al., 2010). Thus, the amount of these components would be critical during embryonic development until hatching. Based on the timing of embryogenic stages, the EP group hatched about an hour earlier than the MP group, judged to be a minor difference, corresponding to their similar egg quality parameters. This suggests that both EP and MP groups can successfully induce hatching to the olive flounder fertilized eggs without delay and time difference between diets.
Genetic information obtained from the broodstock is a crucial element of a fertilized egg, playing a vital role in the normal development of embryos. The expression level of this gene can potentially reduce egg quality or negatively affect post-hatch growth (Reading et al., 2018). Specifically, GH is a growth-related hormone secreted by the pituitary gland after hatching that is also involved during embryogenesis such as gastrulation and organogenesis (Yang et al., 1999). There have been reports of detected GH mRNA expression observed before fertilization and during embryogenesis in Siberian sturgeon (Acipenser baerii) and the mRNA expression level of GH was remarkably increased along with the formation of the pituitary gland in rainbow trout (Abdolahnejad et al., 2015; Li et al., 2006). These results suggest that GH is formed from genetic information received from the broodstock at the beginning of embryonic development. The secreted GH acts on the liver to induce IGF-1 production which plays a key role in growth and metabolism (Hada et al., 2019; Reinecke, 2010). However, studies by Chu & Sadler (2009) and Tao & Peng (2009) have shown that liver formation and function in zebrafish (Danio rerio) occur after hatching. Therefore, it is determined that the continuous decrease in the concentration of IGF-1 in this study is attributed to the non-production of IGF-1 during the embryogenesis of fertilized eggs. In addition, given that Lv is a major component of egg yolk, it is determined that the concentration of Lv will gradually decrease as the egg yolk diminishes after hatching.
During the developmental stage of fertilized eggs, various hormonal contents affecting egg quality are influenced by factors such as photoperiod, water temperature, and the nutritional status of the broodstock (Bobe & Labbé, 2010; Bonnet et al., 2007). In this study, the differences in the content of growth-related factors observed in fertilized eggs produced after the EP diet and those produced after the MP diet are presumed to be attributable to variations in the nutritional status of the broodstock influenced by the feed source. In conclusion, although the effects may vary depending on the diet’s constituents and their quality, the EP diet demonstrated a relatively enhanced effect on improving growth-related factors and development time in olive flounder seed production compared to the MP diet. Therefore, these findings are expected to provide fundamental data on the use of an EP diet to reduce management costs and develop an efficient feeding management scheme for olive flounder broodstocks, ultimately leading to better-quality seed production.








