Introduction
Immune system is a biological defense system that protects the human body from external substances. When this immune system is lowered, it is easily exposed to diseases and various antibiotics are taken to treat diseases. Over the past 70 years, taking many antibiotics has led to the emergence of antibiotic-resistant bacteria (Khan et al., 2021; Khatua et al., 2022). This has led to a shift in treatment strategies toward reducing antibiotic abuse and strengthening the body’s defense system (Khatua et al., 2022; Pezzanite et al., 2021). Boosting immunity is considered a very useful strategy not only for strengthening the body’s defense system, but also for health. In fact, in phase 1, disease is prevented and defected by increasing immunity, but in phase 2, anti-inflammatory agents are used to prevent tissue damage due to excessively enhanced immunity (Mrityunjaya et al., 2020). Nonetheless, enhancing immunity is a very important mechanism for human disease prevention.
Immunity is largely divided into innate immunity and acquired immunity (Netea et al., 2019). Innate immunity refers to a defense system that is present from birth such as skin, mucous membranes, and mucus, whereas acquired immunity is obtained by external substances such as infections and diseases, and antibodies are representative. Immune response is carried out by phagocytic cells as natural killer (NK) cells, leukocytes, and macrophages (Cekici et al., 2000; Netea et al., 2011; Yang et al., 2015). Of these phagocytic cells, macrophages are at the forefront of the immune system and recognize external antigens (Uthaisangsook et al., 2002). Macrophages that recognize the antigen transmit the characteristics of the antigen to helper T cells, and helper T cells self-proliferate and activate cytotoxic T cells to directly kill the antigen (Alberts et al., 2002; Jo et al., 2023). In addition, helper T cells activate B cells to produce antibodies against the antigen, indirectly triggering an immune response. Therefore, the activity of macrophage in immune response is of great importance. Macrophage activity is accompanied by the production of pro-inflammatory cytokines and mediators such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6 and nitric oxide (NO), and these secretions enhances phagocytosis (Bonnardel et al., 2015; Feng et al., 2019; Jo et al., 2023).
Since ancient times, natural resources have been used to treat a variety of human ailments, and more recently have been applied as food supplements due to their functionality. In particular, seaweeds are a valuable source of new bioactive substances and functional foods with potential health benefits, and beneficial physiological activities for anti-inflammatory, antioxidant, and anti-obesity have been reported (Echave et al., 2021; Lomartire & Goncalves, 2022). Codium fragile (CF) is an edible green algae widely distributed in the shores of Asia and Northern Europe, and has been used as an oriental medicine to treat urinary disorder (nocturnal enuresis), enterobiosis and dropsy (Lee et al., 2010; Monmai et al., 2020). CF extracts has been reported to possess anticoagulant, anti-platelet, anti-inflammatory, anti-allergic and chondroprotective effects (Choi et al., 2013; Kang et al., 2012; Moon et al., 2018). It contains 15% protein, 40% carbohydrates, 30% Ash, various vitamins and essential minerals (Table 1). Recently, it has been reported that anionic macromolecules of CF extracted (CFAM) via hot water and ethanol precipitation have an immune-enhancing effect by increasing the expression of nitric oxide, TNF-α, and IL-1β (Monmai et al., 2019, 2020). In this study, we identified the immune enhancing effect of aqueous extract of C. fragile (AECF) and its mechanism, and analyzed the active components responsible for these effects.
| Tests | Results |
|---|---|
| Calorie (kcal/100 g) | 246.4 |
| Carbohydrate (g/100 g) | 41.4 |
| Crude fat (g/100 g) | 3.2 |
| Crude protein (g/100 g) | 13.0 |
| Water (%) | 8.9 |
| Ash (%) | 33.5 |
Materials and Methods
Dried C. fragile was purchased from Wando, Korea in 2021. The sample was ground and extracted in 20 volumes of water (v/w) at 90°C for 4 hours. The extract was spray dried after removing the solvent using a vacuum rotary concentrator. The dried extract was dissolved in growth medium (2 mg/mL) and used for experiments. The general component analysis of CF was commissioned to the Central Life Lab (Seongnam, Korea).
Lipopolysaccharide (LPS), Concanavalin A (Con A), phosphoric acid, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-(1-naphthyl)ethylenediamine dihydrochloride and sulfanilamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kits of IL-6 and TNF-α were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Mouse cytokine array C1 was purchased from RayBiotech (Peachtree Corners, GA, USA). Anti-inducible nitric oxide synthase (iNOS) was purchased from Abcam (Cambridge, UK); and anti-IL-6, TNF-α, NF-κB (p65) and α-tubulin were purchased from Thermo Fisher Scientific; and anti-extracellular signal-regulated kinase (ERK), p-ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38, p-p38 were purchased from Cell Signaling Technology (Danvers, MA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and penicillin/streptomycin were purchased from WelGene (Daegu, Korea). Fetal bovine serum (FBS) was purchased from ATLAS Biologicals (Fort Collins, CO, USA).
Murine macrophage RAW264.7 cells were provided by the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The cells were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin (DMEM growth medium) in a humidified incubator with 5% CO2 at 37°C. For splenocytes isolation, spleens were excised from 2-week-old mice and the harvested spleens were crushed with a plunger on a cell stratiner (pore size; 0.70 µm). After centrifugation at 1,100 g for 10 mins, the cell pellet was washed with 1X phosphate buffered saline (PBS) and then centrifuged. To remove red blood cells, cell pellet was reacted with RBC lysis buffer for 10 mins, and any remaining tissue chunks were removed using a pipette. After centrifugation and washing with 1X PBS, the cells were suspended in DMM growth medium and dispensed at 2 × 105 cells/mL.
Cell viability was analyzed using the MTT technique. The cells were seed at 1 × 106 cells/mL in 12-well culture plate, and then incubated for 15 h. After incubation, the cells treated with AECF (0, 0.25, 0.5, 1, and 2 mg/mL) for 24 h in the presence or absence of mitogen (LPS; 0.2 μg/mL and Con A; 1 μg/mL). And then, 100 µL of MTT solution and allowed to react for 4 h. The formed formazan was dissolved in DMSO and the absorbance was measured at 590 nm, and the survival rate of AECF treated group was calculated by setting the survival rate of the control group to 100%. The experiments were performed in triplicate.
The cells were seed at 1 × 106 cells/mL in 6-well culture plate, and then treated with AECF (0, 0.25, 0.5, and 1 mg/mL) and LPS (0.2 µg/mL) for 24 h. Nitrite accumulated in the medium was analyzed using Griess reagent. TNF-α, IL-6 and cytokines accumulated in the medium were analyzed using ELISA kits according to manufacturer’s protocols.
RAW264.7 cells were treated with AECF and LPS (0.2 µg/mL) for 24 h. Total protein, cytosolic and nuclear proteins were extracted using PRO-PREP solution (iNtRON Biotechnology, Seongnam, Korea) and NE-PER™ nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific), respectively. Protein were quantified using a bicinchoninic acid protein assay kit and equal amounts (20 μg) of protein were separated on sodium dodecyl sulfate-polyacrylamide gels (8%, 10%, and 15%) and transferred to poly-vinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were incubated with specific primary antibodies; anti-iNOS (1:3,000), anti-COX-2 (1:2,000), anti-TNF-α (1:1,000), anti-IL-6 (1:1,000), total/phospho-ERK, JNK, P38 (1:2,000), anti-p65 (1:1,000), anti-IκB-α (1:1,000) and anti-α-tubulin (1:5,000), at 4°C overnight. After washing the membrane with tris-buffered saline containing tween-20, it was reacted with an horseradish peroxidase-labeled secondary antibody (1:5,000) for 1 h. Protein was detected and visualized using an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA) and Micro-Chemi 4.2 imager (DNR Bioimaging Systems, Jerusalem, Israel), respectively.
Results
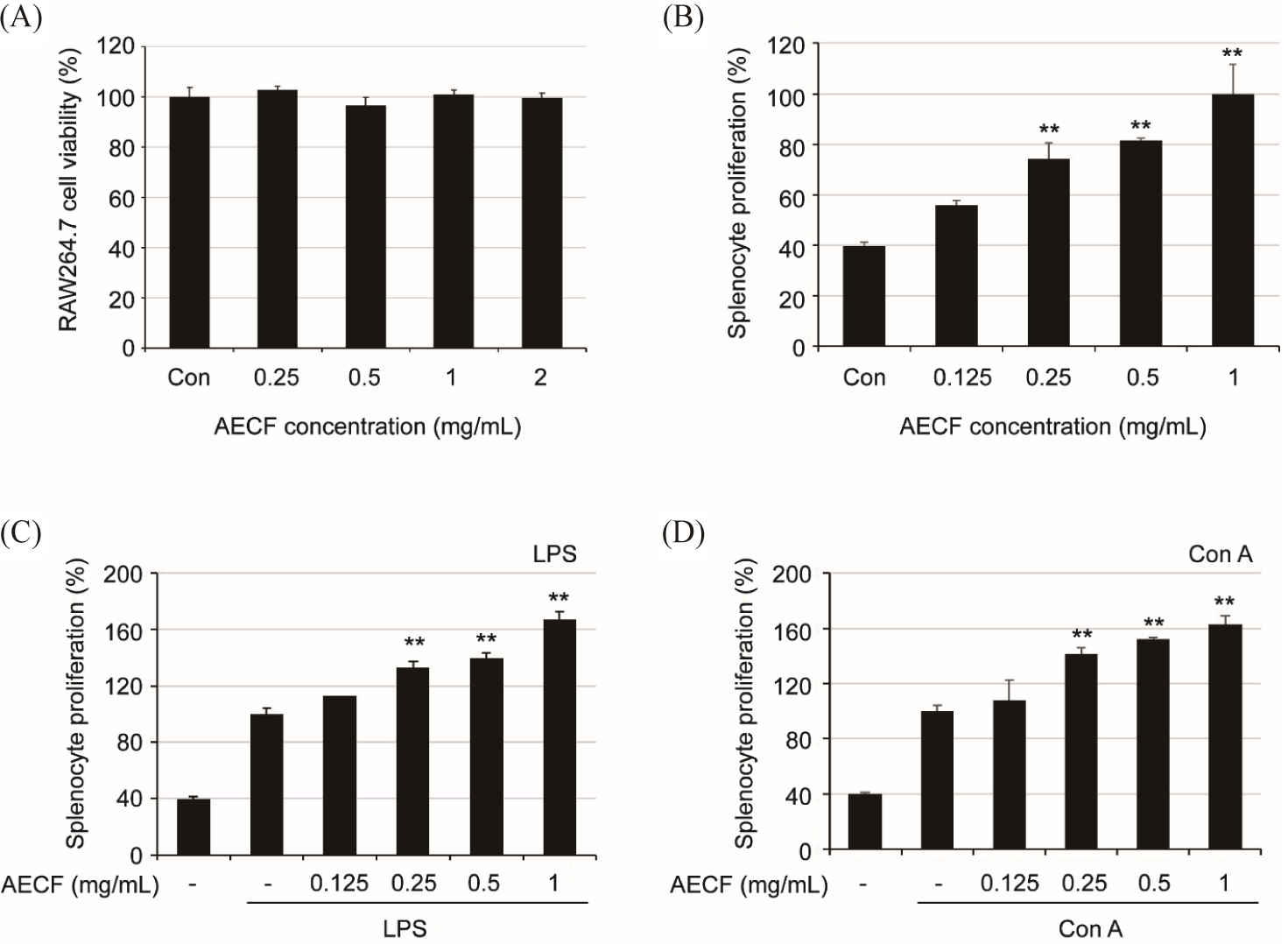
To confirm the cytotoxicity of AECF on RAW264.7 cells, the cells were treated with AECF up to 2 mg/mL, and MTT assay was performed 24 h later. AECF showed no cytotoxicity up to the treated concentration (Fig. 1A). To confirm whether AECF has an immune-enhancing effect, the proliferation ability of isolated splenocytes was evaluated in the presence and absence of mitogen substances (LPS and Con A). As a result, AECF significantly induced the proliferation of splenocytes despite the absence of mitogen (Fig. 1C and 1D). Also, in the presence of mitogen, the proliferation increased more than 1.6 times at 1 mg/mL compared to the group treated with mitogen alone (Fig. 1B).
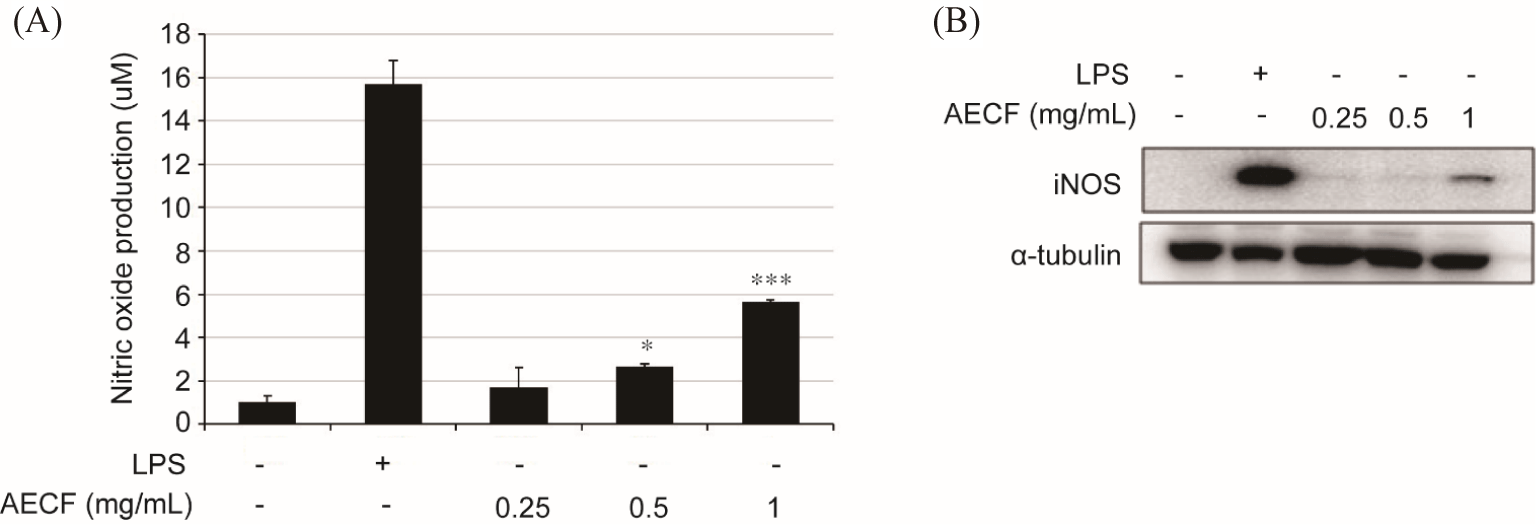
Compared to the control group (1.04 ± 0.27 µm), AECF increased the production of NO in a concentration-dependent manner to 1.69 ± 0.09 µm, 2.68 ± 0.18 µm, and 5.63 ± 1.20 µm at 0.25, 0.5, and 1 mg/mL, respectively, and LPS was 15.68 ± 1.11 µm (Fig. 2A). Also, consistent with the NO accumulation graph by AECF (Fig. 2A), the protein expression of iNOS by AECF gradually increased (Fig. 2B).
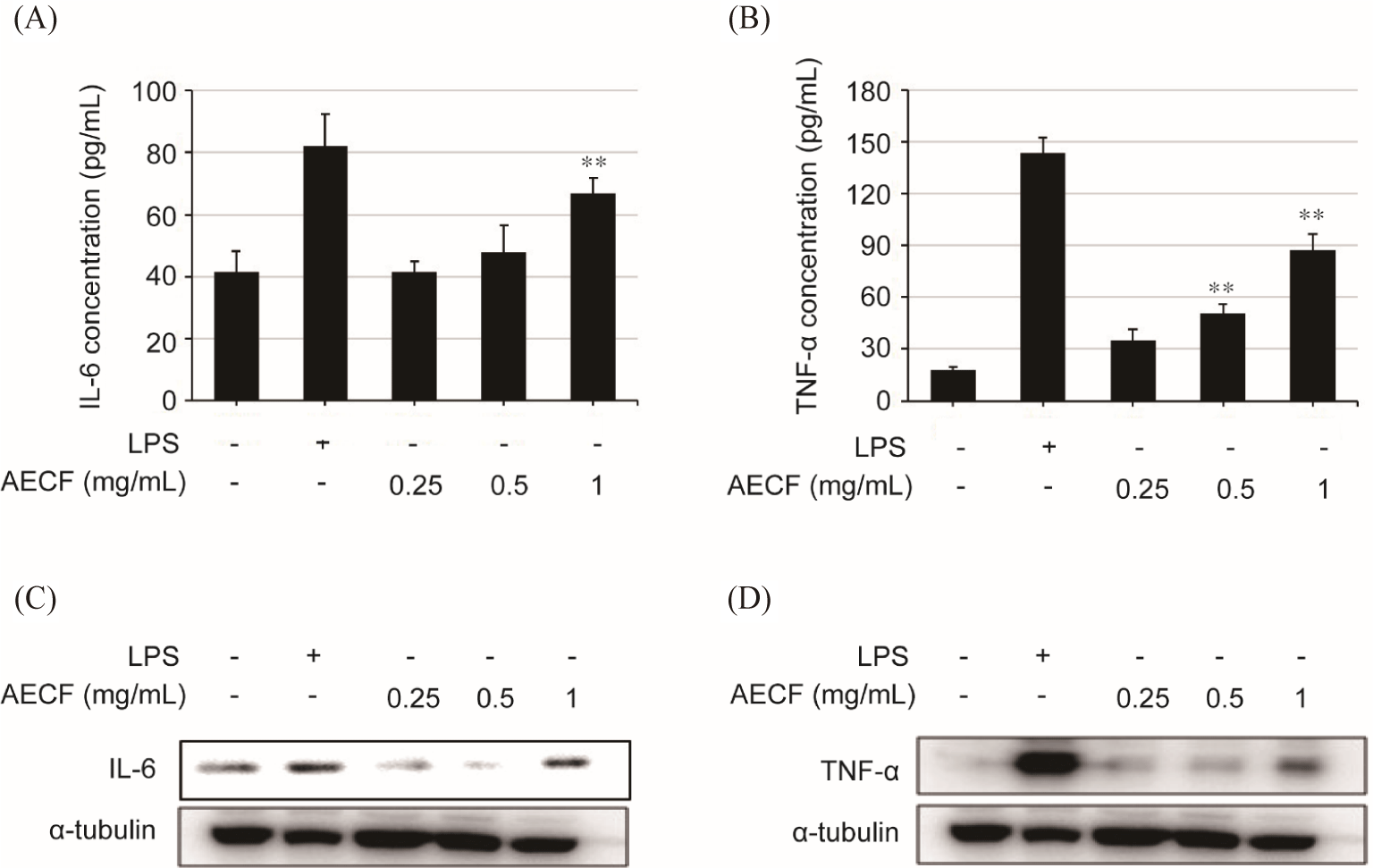
Compared to the control group (41.73 ± 6.37 µm), 1 mg/mL of AECF promoted IL-6 secretion (66.73 ± 5.0 µm). In addition, AECF promoted the secretion of TNF-α in a concentration-dependent manner, with values of 35.06 ± 6.44 µm, 50.9 ± 5.26 µm and 87.56 ± 8.89 µm at 0.25, 0.5, and 1 mg/mL, respectively, compared to the control group (17.73 ± 2.0 µm) (Fig. 3B). These results were consistent with intracellular IL-6 and TNF-α protein expression (Fig. 3C and 3D).
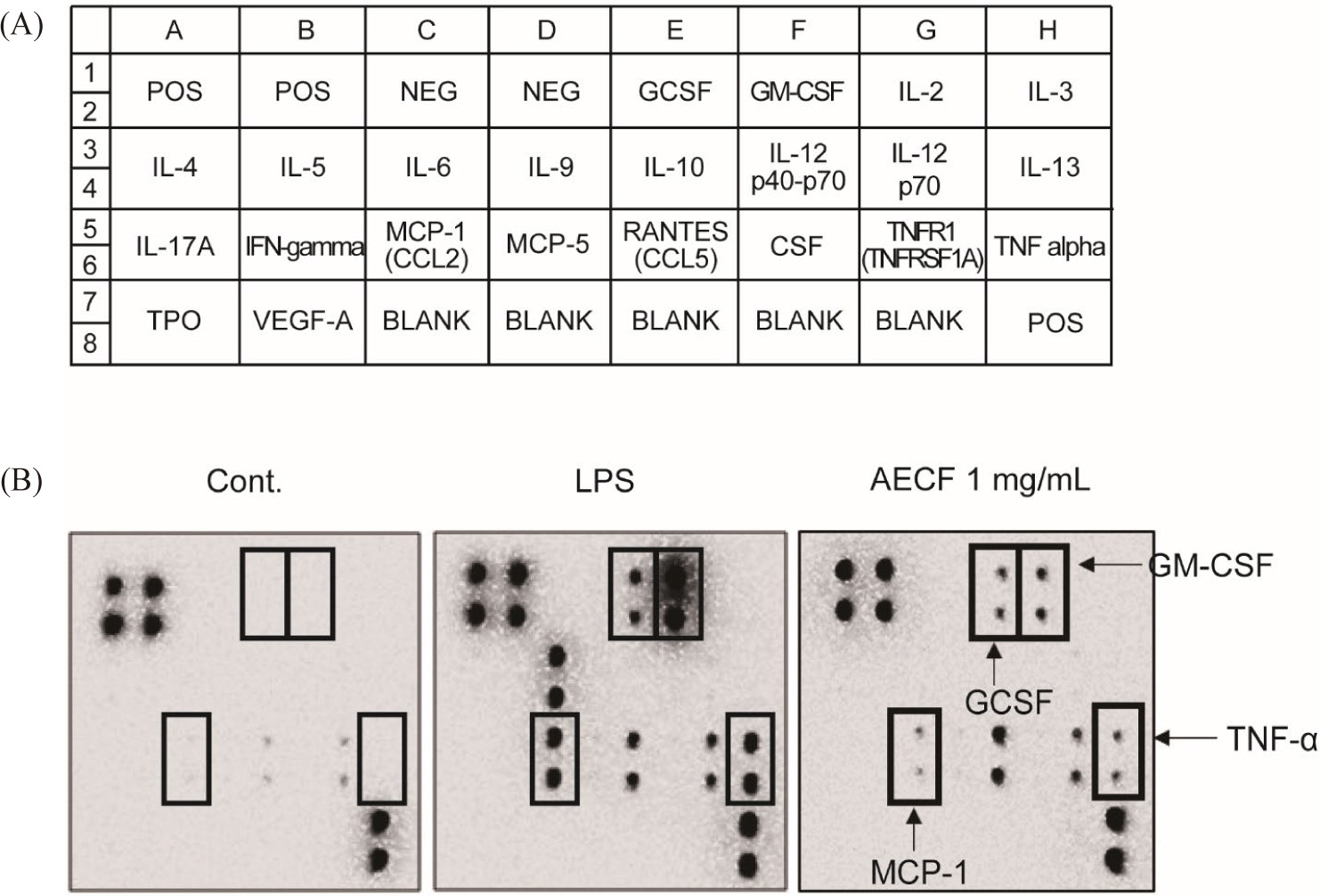
Cytokine arrays were performed to confirm changes in the expression of immune-related factors regulated by AECF. As a result, the expression of granulocyte-macrophage colony-stimulating factor, granulocyte colony stimulating factor, monocyte chemoattractant protein-1, and TNF-α was significantly increased by AECF (Fig. 4A and 4B). These factors promote an increase in the number of macrophages and monocytes and promote phagocytosis, suggesting that AECF exhibits immune-enhancing efficacy through their expression.
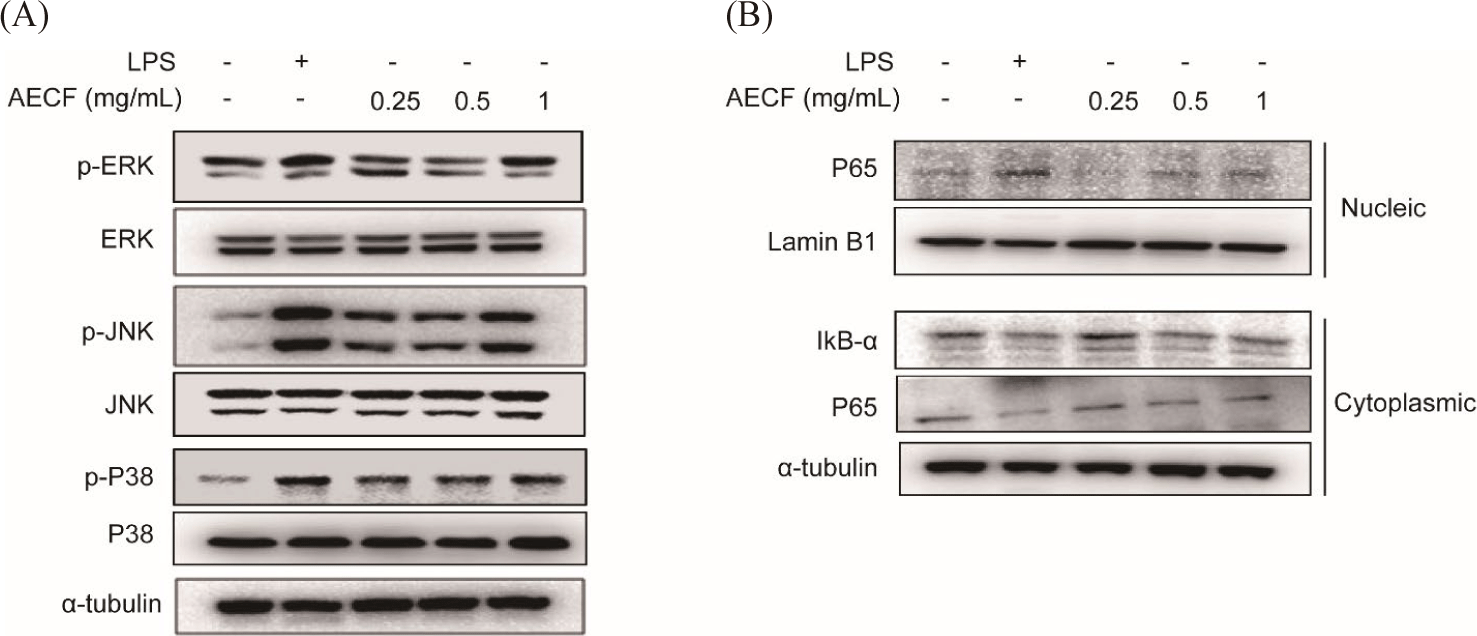
NF-κB and mitogen-activated protein kinase (MAPK) are known to be important mechanisms involved in general cellular physiological activities such as cell survival, development, and differentiation. AECF induced phosphorylation of MAPK (ERK, JNK and p38) in a concentration-dependent manner without affecting the total form (Fig. 5A). In addition, AECF induced phosphorylation of IκB-α, resulting in the degradation of IκB-α and the translocation NF-κB subunit p65 toe the nucleus (Fig. 5B). This immune response of AECF was seen similar to that of the positive control, LPS (Fig. 5).
Discussion
Seaweeds contain large amounts of highly functional polysaccharides such as fucoidan, alginic acid, laminarin, and carrageenan (Lomartire & Goncalves, 2022; Park et al., 2020). In particular, fucoidan is well known to exhibit various functionalities such as enhancing immunity, anti-cancer activity, and anti-blood coagulation, and is often used as a natural health food for the human body (Koyanagi et al., 2003; Park et al., 2020). Fucoidan is a substance obtained mainly from brown algae such as Saccharin japonica and Undaria pinnatifida (Li et al., 2008). Fucoidan is a complex polysaccharide with mainly fucose bound to the sulfate group backbone, and it has been reported that the more sulfate groups there are, the higher the activity on the human body (Li et al., 2008). The CF also contains more than 80% polysaccharide consisting of a sulfate backbone, but unlike fucoidan, its structure is mainly bound by mannose, a water-soluble substance (Park et al., 2020; Surayot & You, 2017). Some studies reported that sulfate polysaccharides or anionic macromolecules isolated from CF are effective in immune enhancement (Monmai et al., 2019, 2020). However, in order to extract these substances, one has to go through complex steps or use solvents that are not food-friendly. Therefore, we attempted to increase the value of CF as a functional material in foods, cosmetics, etc. by extracting the CF with hot water and analyzing its immune-enhancing effect without such a process.
Spleen is a major organ responsible for immune responses, where B and T lymphocytes mature and differentiate (Elmore, 2006). Therefore, proliferation of splenocyte, which contain many immune cells, is considered to be an important indicator of immune enhancement (Drazen et al., 2001). AECF not only induced splenocyte proliferation in the absence of mitogens, but also significantly promoted mitogen-induced splenocyte proliferation. Con A and LPS were used as mitogens for splenocyte, and Con A induces the growth of T lymphocytes and LPS induces the growth of B lymphocytes (Omara et al., 1997). AECF induces proliferation of both T and B lymphocytes, suggesting that it is involved in both humoral and cellular immunity.
Macrophages are one of the most important phagocytes in human immune system and are highly plastic cells that can adjust their profile (M1; classical or M2; alternative) in response to specific environmental stimuli (Lv et al., 2017; Monmai et al., 2020). The activation of M1 macrophages is associated inflammation by secreting iNOS and cytokines to remove invading antigens through a cellular immune response, while M2 macrophage induces an anti- inflammatory response (Lv et al., 2017; Monmai et al., 2020). The iNOS is a pro-inflammatory mediator that fights against antiges by synthesizing NO, which exerts an immune effect by incresing microbial damage (Apetoh et al., 2007). In addition, pro-inflammatory cytokines, such as IL-6 and TNF-α, are mainly secreted by activated immune cells and protect the body from antigens by regulating inflammation and immune response for anti-bacterial and anti-viral (Apetoh et al., 2007; Seo & Shin, 2012). The Euglena gracilis group showed a similar pattern of increased NO production and TNF-α in RAW264.7 cells, suggesting that the results of increased iNOS, NO, IL-6 and TNF-α production by AECF induce immune enhancement.
MAPKs and nuclear factor-kappa B (NF-κB) are representative signaling systems that regulate a variety of biological responses within cells, including cell growth, survival, and differentiation as well as immune and inflammatory stress (Chen et al., 2023). Previous studies showed that Giardia duodenalis extracellular vesicles promotes the secretion of pro-inflammatory cytokines through MAPK and NF-κB signaling activity in mouse macrophages (Zhao et al., 2021). In addition, berberine, known as natural inflammatory regulator, was shown to exert immunoactivities through the MAPK, NF-κB, and JAK/STAT signaling pathway in the immune system (Haftcheshmeh et al., 2022). Consistent with the results above, AECF promoted the secretion of pro-inflammatory cytokines and the expression of factors that support immune activity through activation of the MAPKs and NF-κB signaling pathway in RAW264.7 cells.
Conclusion
The present study demonstrated that AECF enhances immunity by releasing the expression of pro-inflammatory cytokines and immune-stimulating factors through the MAPK and NF-κB signaling pathways. In addition, AECF significantly induced the proliferation of mitogen-free splenocytes and also affected the proliferation of T and B lymphocytes. Therefore, these results indicate that hot water extract of CF can show significant efficacy on immune enhancement without the complicated process of sulfated polysaccharide extraction of CF. AECF has been confirmed to contain large amounts of many free sugars, including barssicasterol and phytol, and further research is needed into the active componenets that exhibit immune activity (data not shown). In addtion, further analysis of phagocytic activity of splenocyte by AECF will need to be performed to identify the exact mechanism and efficacy of the immune-enhancing effects of CF.







