Introduction
Scylla is a genus of mud crabs that can be found all over the world, from the coastlines of Southeast Africa to the Indo-Pacific (Fazhan et al., 2021). In Southeast Asia, the purple mud crab Scylla tranquebarica is among the species in the genus Scylla that is highly valued and very popular in China, Indonesia, Malaysia, Singapore, Thailand, Philippines, and Vietnam (Cheng et al., 2022). This mud crab is one of the luxury foods in very high demand in the international market (Jumawan et al., 2021).
Despite its popularity so far, it turns out that the wild stock of mud crabs has been severely depleted because of fishing pressure to meet export demand (Jumawan et al., 2021). Furthermore, recent initiatives to promote mud crab aquaculture have relied exclusively on seeds taken from the wild, which has prompted concerns about sustainability. As a result, the Indonesian government has regulated mud crab catches through the Ministry of Marine and Fisheries while encouraging seed production and pond culture development.
Mud crab breeding in captives has been investigated recently to meet the demand for seed crab farming (Waiho et al., 2018). The purple mud crab has undergone extensive exploitation and study, however, the aggressive behavior of mud crabs during the nursery stage, which promotes cannibalism and lowers the survival rate (SR), continues to pose a concern for their growing performances (Ikhwanuddin et al., 2015).
The use of shelters and density restrictions have been tried to reduce cannibalism in crabs (Yin Thien et al., 2022) however, cannibalism has not been completely abolished. Utilizing fresh feed, such as trash fish, mollusks, and squid meat for growth and to cope with cannibalism has been practiced with good results, however, facing an issue with their availability (seasonal), storage, and nutrient leaching. As for pellets, there is a problem with their palatability. Therefore, both types of feed often cause problems of decreasing water quality and spur the development of pathogenic bacteria (Shao et al., 2013; Shelley & Lovatelli, 2011). Unlike other crustaceans, the crablet stage of mud crabs in the nursery prefers live feed more than artificial feed (Eastman & Thiel, 2015). This is in line with the feeding habits of mud crabs which are scavengers as well as generalist predators that actively hunt and consume a wide range of small invertebrates, including crustaceans, gastropods, and bivalves, in their natural habitat (Liew et al., 2022). Hence, employing live feed in the form of amphipods can serve as a viable option to enhance growth performance and mitigate cannibalism in hatcheries.
Amphipods possess the ability to endure diverse climatic conditions, exhibit rapid reproduction, a short lifespan, and ease of maintaining in high populations throughout the year so it is well-suited for utilization as live feed in aquaculture (Baeza-Rojano et al., 2012). Nevertheless, there have been no reports on amphipods used as the main source of food for mud crab genus Scylla. However, in our previous research, we examined the utilization of amphipods as live food for tiger shrimp juvenile Penaeus monodon (Sulaeman et al., 2020) and blue swimmer crab Portunus pelagicus crablet (Herlinah et al., 2020) with encouraging results. Prior studies have also investigated the use of amphipods as a main food source for octopus (Octopus sp.) (Baeza-Rojano et al., 2012), spiny lobster (Wang & Jeffs, 2014), and pink shrimp (Corona et al., 2000), yielding positive outcomes.
The proximate study revealed that specific marine amphipods exhibit diverse protein levels. Belmos quadrimanus sp. was discovered to possess a protein content of 37% (Promthale et al., 2021), Hyale perieri exhibited 44.6% (Baeza-Rojano et al., 2014), and marine amphipods, demonstrated a protein level of 52.4% (Opstad et al., 2006). Nevertheless, these protein levels are lower in comparison to adult Artemia, measuring 55.28% (Anh et al., 2010). In addition, amphipods possess a higher concentration of fatty acids. According to Baeza-Rojano et al. (2014), amphipods have an EPA fatty acid concentration of 17.7%, while adult Artemia, as reported by Léger et al. (1986), has a content of 5.9%. Furthermore, Baeza-Rojano et al. (2014) observed that amphipods contain 13.6 DHA fatty acids, but Ma et al. (2023) found that adult Artemia only has 0.09%. However, the concentrations of crude fats and essential amino acids are comparable in both scenarios, as evidenced by the studies conducted by Baeza-Rojano et al. (2014), Jiménez-Prada et al. (2018), and Léger et al. (1986, 1987). An important aspect to notice regarding amphipods is their significant accumulation of heavy metals, including cadmium (Cd), which can reach concentrations as high as 12 mg/kg. This concentration is six times greater than the upper limit set by the European Union (Moren et al., 2006).
The current study is the first to investigate amphipods as a live feed for the mud crab S. tranquebarica. The preying capacity shown in daily consumption and the growth performance is a necessary and important parameter when introducing new feed in aquaculture as basic information to estimate feed requirements and determine feasibility for daily practice (Vargas-Abúndez et al., 2021). Therefore, this study examined the preying capacity of the crablet stage of mud crabs, S. tranquebarica exposed at different amphipod densities and elucidated their growth performance. The use of amphipods as an alternative feed can be a solution to reduce cannibalism and improve growth performance, especially in larval and nursery rearing.
Materials and Methods
According to the Chattogram Veterinary and Animal Sciences University’s ethical and legal clearance committee, animal ethics approval is not required for crustaceans, including the Portunid crab (S. tranquebarica), because it is an invertebrate (Rahman et al., 2020). As a commercially available crab species, S. tranquebarica does not require any special license to acquire in the country. However, we followed the Australian Code of Practice standards for the care and use of animals in scientific research in this work (Anonimous, 2013).
The healthy wild female broodstocks of mud crab S. tranquebarica were collected from mud crab landing in Malili regency, South Sulawesi, Indonesia. The procedure of broodstock handling, larvae, and juvenile rearing under laboratory conditions referred to Gunarto & Parenrengi (2014) in preparing the crablets used in this study. The crab larvae were cultivated in conical-shaped fiberglass tanks, each of 250 L in volume. Each tank is filled with sterilized seawater at a salinity of 30 ppt. Larvae were fed HUFA (highly unsaturated fatty acid) enriched rotifer (Brachionus sp). As larvae grow the food changes to HUFA and nannochloropsis enriches Artemia nauplii from larvae zoea-3 to the megalopa stage. Once they reach the crablet stage, they are fed on fresh feed such as fish, squid, mollusk, and shrimp meat or dried shrimp (Acetes sp.) to excess served twice a day until used.
A total of 45 glass jars with individual crablets of similar size were randomly assigned to the three different amphipod densities. Thus, 15 jars were randomly assigned to each treatment (n = 15). This experiment utilized a glass jar with a height of 23 cm and a diameter of 15 cm filled with 0.5 L of disinfected seawater. Each container has moderate aeration. All jars were placed in the incubator tank with heaters to keep the water temperature consistent throughout the trial. The experiment was hosted in a semi-proof laboratory building and subjected to a 12 h light:12 h dark cycle of natural light. The water medium was exchanged 100% at the time of feeding in the morning and evening.
The amphipod used in this experiment was identified as Grandidierella megnae (Giles, 1890) (Herlinah et al., 2020) collected from mass culture in an indoor concrete tank at the research installation of the Research Institute for Brackish Water Aquaculture and Fisheries Extension (RIBAFE) Barru, Indonesia. Only the adult individual (body weight, 0.00059 ± 0.00006 g) was selected and used in the experiment. The amphipod was selected by first passing them through a scope net of appropriate pore size to obtain large numbers of animals of similar size. Live feed was provided once a day for the first ten days, then twice a day the next day (morning and evening). The amphipods harvested from mass culture are then counted and placed in a 250 mL plastic cup container before being delivered into the crablet jars according to their respective treatments. The amphipod density tested was as follows: A: 20 amphipods / 0.5 L, B: 30 amphipods / 0.5 L, and C: 40 amphipods / 0.5 L. The remaining amphipods in the morning and evening were counted and removed from the container before the next feeding, and the number of crab molting was recorded.
The preying capacity of crablet on a live amphipod is explored by measuring different parameters. Overall consumption rate (OCR) is simply the percentage of the number of amphipods consumed during the experiment calculated as OCR (%) = (number of prey consumed/number of given) × 100. Daily consumption rate (DCR) is calculated according to the formula DCR (%) = (total prey consumed (g) / {(W2 + W1 × 100) / 2} × t (day). Where W1 = initial weight and W2 = final weight. The DCR were presented for 30 days and 60 days rearing periods as DCR-30 and DCR-60 respectively. The same formula was also used to calculate the daily consumption rate during daytime (DaCR), night (NCR), the first month, and the second month by simply replacing the total prey consumed with daytime, nighttime, the 1st 30-days, and the 2nd 30-days consumption respectively. While feed efficiency ratio (FER) is calculated using the formula: Feed conversion ratio (FCR) = amphipod consumption (g) / W – gain (g).
Before stocking, the weight (W) was determined with an electric scale (Camri) to the nearest 0.01 g. The W was recorded for each crab every ten days and then transferred back to the respective jar to determine the trend in crablet size development during the experiment.
The specific growth rate of W was calculated as SGR-W = (ln W2 – ln W1) / t × 100% where W1 = initial weight (g), and W2 = final weight (g), SGR-W = Specific Growth Rate for the weight (% / day), and t = rearing duration (days). The percentage weigh increment is = (postmolt W – premolt W) / premolt W × 100. The specific growth rate of weight (SGR-W) were presented for 30 days and 60 days rearing periods as SGR-W-30 and SGR-W-60 respectively.
The survival rate is simply the percentage of a survivor at the end of the experiment and calculated according to SR = Nt / No × 100%, where SR = Survival Rate (%), Nt = the number of survivors at the end of the experiment, No = is the initial number of crab. In the same way, the molting rate or cumulative molting is calculated as CM = (Mo / C) × 100, where Mo = Molting occasion during the experiment and C = The number of crablets (crab) per treatment (Nguyen et al., 2014).
Water quality observations were carried out daily, including temperature and salinity, while dissolved oxygen (DO) and ammonia were carried out at the beginning and every ten days of rearing. The temperature was measured using a Hobo data logger (Onset, Bourne, MA, USA), salinity with a hand refractometer (Atago), dissolved oxygen with a DO meter (YSI Pro 2030), and ammonia with a test kit (T617, Tetra, Pasadena, CA, USA).
Experimental data of OCR, DCR, FCR, SR, cumulative molting (CM), and SGR-W parameters derived from fifteen individuals were analyzed in a one-way ANOVA to evaluate the variance in amphipod density. One-way ANOVA was used to assess parameters generated from fifteen different individuals. The Shapiro-Wilk and Levene tests, respectively, were used to verify the normality of residuals and homogeneity of variances assumptions made by ANOVA. Using IBM-SPSS 21 (IBM, Armonk, NY, USA), a statistical analysis program, multiple comparisons Duncan tests were produced. On the other hand, the DCR differences between the first and the second month of the rearing period also the DaCR and NCR were compared using a t-test. Water quality parameters (salinity, temperature, DO, and ammonia) were presented descriptively.
Results
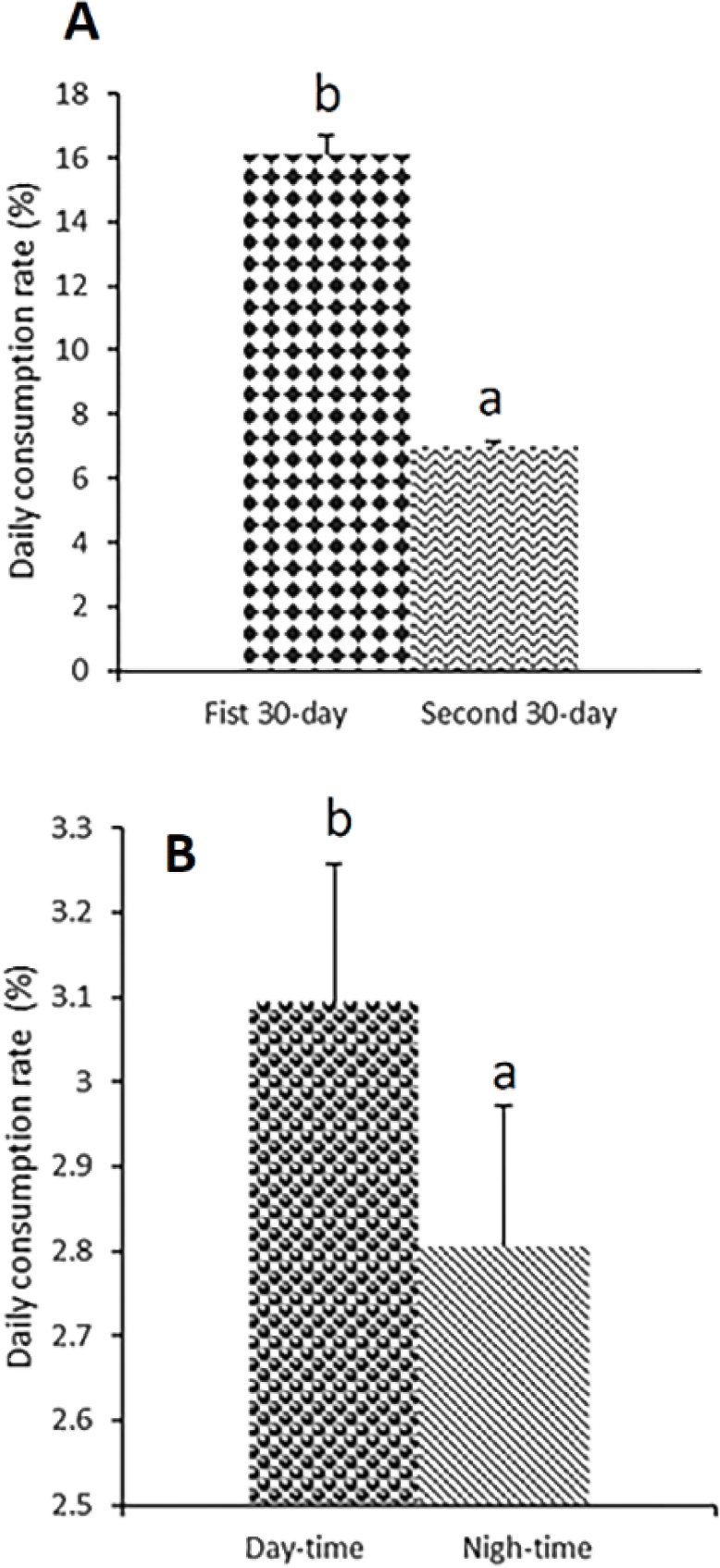
The feeding activity of the crablet on the amphipod was regularly examined during the experiment and confirmed that the crablet voluntarily preys on the amphipod immediately after being served. A crablet can eat an amphipod in less than a minute and eat the entire given amphipod in just a few hours, especially in low amphipod density (20 amphipod / 0.5 L). The higher the stocking density, the lower the OCR observed (Table 1). There was a significant difference in OCR between crablets from different stocking densities of amphipods (p < 0.05). The highest amphipod consumption (96.89%) was found in treatment A, which was not significantly different from treatment B (95.03%), but it was higher and significantly different from treatment C (91.98%). In contrast to OCR, the DCR-60 was not significantly different (p > 0.05) with an increase in the stocking density of the amphipod (Table 1). The crablet preyed on amphipods in the range of 8.11%–8.55% body weight per day during the study regardless of the density of the amphipod given. Different from DCR-60, DCR-30 showed significant differences (p < 0.05) where treatment B and C were statistically equal, but they were both higher than treatment A (Table 1). The preying activity of crablet was also influenced by a light-dark condition where the DaCR (3.02 ± 0.31%) was significantly higher (p < 0.05) than NCR (2.72 ± 0.59%) as shown in Fig. 1B. It may indicate that preying activity is affected by the visibility of prey. As the crablet grew, DCR declined significantly (p < 0.05). The DCR-60 (6.98 ± 0.17) was significantly lower compared to DCR-30 (16.08 ± 0.61) (Fig. 1A). Although the preying capacity of crablet against amphipods varied in different treatments, the FCR did not show a significant difference (p > 0.05) (Table 1).
The molting frequency of crablets during the experiment is presented in Table 1. The higher the amphipod density, the higher the molting frequency. The highest overall crablet molt from 3–5 times the experimental period. Amphipod densities significantly affected the molting frequency (p < 0.05). The molting frequency at treatment C (583.33 ± 16.67) did not differ significantly from treatment B (555.56 ± 8.01), but both were significantly higher than A (473.33 ± 6.67).
The survival rate of crablets during the experiment is presented in Table 1. No crab death events were encountered during the study or with 100% SR for all treatments. Based on statistical analysis, the difference in the density of the amphipods given did not have a significant effect (p < 0.05) on the SR of mud crab.
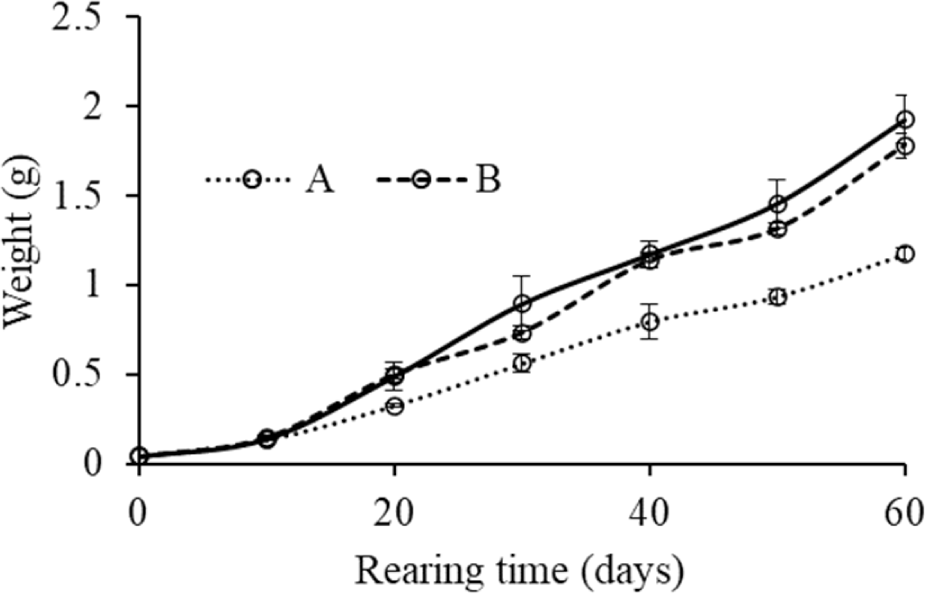
The growth performance and feed utilization of purple mud crab S. tranquebarica fed different stocking densities of amphipod for 60 days are shown in Table 1. During the feeding trial, the W of mud crab grew from 0.04 g to 1.17, 1.81, and 2.12 g from the lowest to the highest prey density, respectively. The increase of W from the beginning to the end of the study is visualized in Fig. 2. The W differences between treatments began to be seen at the 20-day rearing period and were sounder at the end of the experiment.
The growth performance expressed as SGR-W after 30 and 60 days of different amphipod density treatments is presented in Table 1. In line with the W, the SGR-W also increased when the amphipod density increased. Based on statistical analysis, the difference in amphipod density had a significant effect (p < 0.05) on both the SGR-W. The highest SGR-W was found in treatment C, which was not significantly different from B, but both were significantly higher than A in both 30-day and 60-day rearing periods. Different feeding intensities also have a significant effect on the average percentages of weight increment (Table 1). The crablet receiving the lesser number of amphipods showed significantly lower values compared to the other groups (p < 0.05).
Water quality may affect the molting process and growth performance during crab cultivation. The results of water quality measurements, including temperature, salinity, pH, dissolved oxygen, and ammonia observed during the experiment, are presented in Table 2. Water temperature and salinity are among the important water quality parameters that need to be addressed during the whole life cycle of mud crabs because directly related to the physiological process of their growth (Syafaat et al., 2020). The range of temperature during the study was between 27°C–31°C and lower compared to the range suggested by Syafaat et al. (2020) at 28°C–32°C. On the other hand, changes in salinity due to evaporation in water reservoirs and rearing containers resulted in a salinity range of 29–32 ppt. The range was still within the range suggested by Baylon (2010) at 20–35 ppt.
| Parameters | Value range | Optimum range | Reference |
|---|---|---|---|
| Temperature (°C) | 27–31 | 28–32 | (Syafaat et al., 2020) |
| Salinity (ppt) | 29–32 | 20–35 | (Baylon, 2010) |
| DO (ppm) | 5.05–5.24 | 5 < | (Shelley & Lovatelli, 2011) |
| Ammonia (ppm) | 0–0.25 | < 3 | (Shelley & Lovatelli, 2011) |
The capacity to breathe air allows mud crabs to successfully use their habitat even at low tide and exit water with low oxygen levels. The DO content measured in the water medium ranged from 5.05–5.24 mg/L is still an appropriate value as described by Shelley & Lovatelli (2011). Another important water parameter to mention is ammonia. The high ammonia content could affect growth and cause death (Waiho et al., 2018). The ammonia content during the study was around 0–0.25 ppm, which is lower than recommended by Shelley & Lovatelli (2011) at < 3.0 ppm.
Discussion
Appropriate feeds and feeding regimes are critical to hatchery success and are frequently complex and species-specific (Syafaat et al., 2021). In mud crabs, claws have been formed at the megalopa stages, making it easier to catch their prey. In this weaning experiment, adult amphipods are highly favored by crablets where they continuously actively prey until they reach a high OCR (91.98%–96.89%). There was a general trend of increasing preying capacity with the increasing density of amphipods. A significantly large number of amphipods consumed was obtained at the highest density of prey (40 amphipods / 0.5 L) and the least consumed was at the lowest density of 20 amphipods / 0.5 L. This is certainly reasonable because the more available prey, the greater the chance of consuming more. This is in line with previous results where the total amount of Artemia ingested rises with the increase of prey density in Scylla paramamosain (Zeng & Li, 1999) and Panopeus herbstii (Harvey & Epifanio, 1997) during the early developmental stage.
It is important to mention here that most of the time during the experiment, the whole amphipod was ingested without excess at low density but always left at high density. At medium density in treatment B (30 amphipods / 0.5L) becomes the ideal density in terms of feed efficiency where the day amphipods remain and run out is balanced. Although amphipod stocking density had a significant effect on OCR and DCR-30, the DCR-60 showed no significant difference (p > 0.05). This may be because the increase in the ability to prey as the crablet grew was not followed by the addition of amphipod availability particularly at the lower amphipod densities.
When the DCR data were pulled together across the amphipod stocking density and compared between the DCR-30 (1st 30-day) and the DCR-60 (2nd 30-day) it showed a significant decrease (p < 0.05) as shown in Fig. 1A. In aquaculture, it is common for the predation capacity to decrease as they grow larger (Corona et al., 2000). The preying capacity during the juvenile stage often initiated with a higher feeding capacity before then decreasing relative to their size as they grew (Baylon et al., 2003). One possible explanation for this phenomenon is related to energetic efficiency. Juvenile organisms are typically more energetically efficient in capturing and processing prey due to their smaller size and higher metabolic rates. They can often capture and convert a higher percentage of the energy from prey into growth and development (Baeza-Rojano et al., 2012).
Mud crab is classified as predator and scavenger crustacean which turn to cannibalistic. The crablet’s predation capacity against amphipods is also affected by the time of predation. When DCR data are put together according to feeding time i.e., day and night regardless of amphipod feeding density, it also presents interesting data. The DaCR (morning feeding) was markedly higher than NCR (evening feeding) even if fed the same number of amphipods (Fig. 1B). This may indicate that crablet predation on amphipods is influenced by visual detection factors supporting mechanoreceptor function located on the antennae and or hairy legs (Baylon et al., 2003). This may imply the need to consider applying more amphipods during the day compared to night as opposed to the habit of doing otherwise for nocturnal species. Crustacea feeding activity varies during the day and night, which may be due to internal factors adapted to photoperiodic and feed availability (Gomes do Vale et al., 2022). This is in line with the finding of Alberts-Hubatsch et al. (2016) that the juvenile of S. serrata is nocturnal. In contrast to the results of previous studies (Kamaruddin et al., 2016) which showed that the feeding rate of crablets on pellets was higher at night compared to during the day. It is believed that the crablet feeding on live prey in this study is highly dependent on light availability besides the chemoreceptor that is mostly used to locate the prey during the night. On the other hand, crablet may use a chemoreceptor when locating pellet that does not require visibility. Some catfish that mostly located food by their chemoreceptor, experienced better growth rates when they were fed during nighttime, for example, African catfish Heterobrachus longifilis (Bolliet et al., 2001) and Heteropneuster fossils (Kujur et al., 2021). Although amphipods as a live feed that can be co-cultured with crablets, higher stocking density in the daytime or light introduction during nighttime needs to be considered, however, more detailed investigations are still needed in the future.
The daily feed intake is an important parameter that can be used to predict the amount of feed that should be allocated daily in rearing practice. Different from young finfish where the live prey items need to be swallowed whole and must be of an appropriate size, crablets could prey on amphipods whose size is equal to or larger than their carapace size. The daily consumption rate ranged from 8.02%–8.55% / day in this study was in the range suggested at the ranges of 3%–5% / day (Usman et al., 2016). Undifferenced prey consumption at different stocking densities of amphipods as described above was also reflected in statistically equal FCR in this study. This means that the lower the density of the prey, the more efficient the application. However, since the growth performance of the A treatment is inferior, the B treatment is the most appropriate to be applied.
The growth performance of crablet feeding on different densities of amphipod was variable. The lowest SGR-W in treatment A compared to other treatments is believed to have arisen because of insufficient feed to increase the growth of mud crab’s juvenile. Growth can be achieved when the energy acquired and conserved exceeds the energy expended on biological processes, through the availability of an adequate supply of food (Catacutan, 2002). Indications of lack of food in treatment A can be seen in eating activities where often the feed given runs out in just a few hours and the OCR was higher (96.89%) compared to B and C treatments. This underfeeding condition has led to growth stunting and failure to attain desirable sizes as commonly occurs in aquaculture species (Aaqillah-Amr et al., 2022). This is relevant to the previous finding that the intermolt duration of S. paramamossain juvenile was significantly extended while W gain of newly molted crabs was significantly reduced when the feeding rate decreased (Gong et al., 2022). However, SGR-W in the 1st 30-day (9.66%) in this experiment was higher compared to 7.75%–8.27% in Scylla olivacea fed on dry pellet (Kamaruddin et al., 2016; Usman et al., 2016) and in the range of the finding of Anh et al. (2010) at 8.85%–15.21% by the used of Artemia on S. paramamosain. Even up to the 60-day rearing period, the SGR-W obtained here (5.61%–6.66%) is still higher than the results obtained by Fatihah et al. (2017) (5.07%) when crablet fed on the fish meat and squid.
The highest SGR-W was obtained in treatment C presumably because the amount of feed given could meet the nutritional needs of crablet to grow where the energy stored was more significant than the energy used for body activities. However, the decrease in the percentage of amphipods consumption in treatment C (91.98%) from A (96.89%) may provide a clear indication of overfeeding. Since the growth performance of crablet in treatment C did not differ significantly from treatment B suggested that B could be the best choice and suitable to apply in terms of feed efficiency. Overfeeding may result in the accumulation of unconsumed feed, in which dead amphipods may rapidly decay, affecting water quality in the culture medium (Shao et al., 2013) and potentially causing the emergence of pathogenic microorganisms and even the death of cultured organisms. Underfeeding and overfeeding both have negative economic and environmental consequences. So, feeding control and determination of the optimum feeding rate are essential to the success of the fish culture (Shao et al., 2013).
Somatic tissue growth occurs when crustaceans routinely shed their old exoskeleton (molting) which causes a slight rise in body size. Because molting in crustaceans is a function of growth (Chang & Mykles, 2011), the molting frequency can be used as a growth indicator in crablets. The duration of the molt cycle is the culmination of a complex mechanism involving both exogenic and genetic components (Heasman, 1980). However, the general pattern shows that growth rates consistently decline when the food supply falls below ideal levels (Ruscoe et al., 2004). Extended intermolt intervals and decreased molting increments are also contributing factors to this decrease. The data provided in this study supports that trend where the higher the number of amphipods given, the higher the DCR and SGR-W.
In line with the growth performance described above, the molting frequency in this experiment increased when the amphipod density increased, with the range of molting frequency of 4–6 times during the experimental period. The molting frequency was significantly affected (p < 0.05) by amphipod densities where the molting frequency at treatment C (583.33 ± 16.67) did not differ significantly from treatment B (555.56 ± 8.01), but both were significantly higher than A (473.33 ± 6.67). In line with the frequency of molting, the weight gain in the last molting case also showed a marked difference (p < 0.05) with a range of 71.61%–94.42%. The weight gain at treatment B (94.42 ± 6.92%) did not differ significantly from treatment C (89.01 ± 3.99%), but both were significantly higher than A (71.61 ± 2.39%). The increase of W in this experiment is in the range of the previous finding (Anh et al., 2010), especially at the fifth molting stage (60%–140%). The proper completion of the extremely complicated process of molting requires careful coordination between different functions/systems in the body. Therefore, success in molting may indicate that the whole performance of a respective individual is good. However, no detailed data was provided related to the health condition of crablets in this experiment.
The main causes of mortality of fish in mass culture are food deficiency, predation/cannibalism, unfavorable water qualities, and pathogens. In principle, these can be eliminated indoors under controlled culture conditions with sufficient exogenous feed supplied (Syafaat et al., 2021). The amphipod density applied in this experiment would, at least in part, clarify the absence of negative effects seen in prior investigations on shrimp (Sulaeman et al., 2020), and swimming crabs (Herlinah et al., 2020). The adequacy of feed intake for all treatments showed optimal conditions where no mortality occurred during the experiment. This follows the statement of Kamaruddin et al. (2016) that the SR of mud crab juveniles (Scylla serrata) can be improved by the provision of food availability. Successful feeding trials with amphipods have also been reported earlier such for blue swimmer crab P. pelagicus (Herlinah et al., 2020) and tiger prawns P. monodon (Sulaeman et al., 2020). Given that they were acclimated to live feed with Artemia during the megalopa stage, may have made it quickly adjust to live amphipods. Other advantages of live-feed amphipods in this case over other diet types are the ability of the former to remain alive and come around in the water and their plasticity of shape which permits crablet to ingest them more easily. The application of living prey in lobster and marine fish (Johnston et al., 2008) can reduce cannibalism greatly.
In conclusion, the preying capacity of mud crabs increased with the prey density. However, it was significantly reduced from the 1st 30 days to the 2nd 30 days of the rearing period and from daytime to nighttime feeding. With this preying ability, the parameters measured including SGR-W, CM, SR, and FCR all demonstrate the excellent amphipod potential to be used as live feed for mud crab nurseries. In particular, the stocking density of amphipods of 30 amphipods / 0.5 L can be recommended as the optimal density to optimize the DCR and improve the growth performance. It is also proposed that surplus live feed will potentially reduce the cannibalism rate of S. tranquebarica at the crablet weaning. However, more research needs to be carried out to elucidate the benefits of crablet-feeding amphipods in communal systems. The results of this study are expected to be a piece of basic knowledge for future research and new information for crab hatchery actors in overcoming the difficulties of choosing the right and easy type of feed.
