Introduction
The genus Chirostoma includes charales and white fish, they are endemic from the Mesa Central of Mexico; this one is composed of 18 species and 6 subspecies from Atherinopsidae, controversial taxon in terms of its origin, colonization and diversification in the Altiplano Mexicano (Barbour, 1973). It is discontinuously distributed in lentic systems, along the Lerma River Basin (Hernández-Rubio et al., 2006). This basin is characterized by a high degree of urbanization and industrialization, habitat fragmentation and exotic species introduction, that have decreased their fish populations size and others one are in some category of risk (SEMARNAT, 2019).
Chirostoma jordani (Woolman, 1894) has the major distribution of the jordani group (Barbour, 1973; Miller et al., 2005). It inhabits in clear and turbid waters from several lakes, rivers channels and dams, whit great load of sewage waters and xenobiotic contaminants that negatively affect biochemical and physiological functions of aquatic fauna, besides of insecticides, agrochemicals and fertilizers derived from agricultural activities (López-López et al., 2006).
C. jordani, because of its wide distribution and not be in any risk category, in agree NOM 059-SEMARNAT (2019), it can be used as model to implement conservation strategies, applicable of five species in risk of Chirostoma (C. bartoni, C. charari, C. labarcae, C. promelas and C. riojai). Sperm cryopreservation is inside these conservation strategies. Because of this technique allows to maintain cell viability and functionality at low temperatures, it has been recognized as an effective method for assisted reproduction in multiple fish species, either in captivity or in some risk category (Viveiros et al., 2015).
Fish semen cryopreservation involves mixing it in diluents that prevent motility and cryoprotective solutions, whit osmotic and nutritional properties, which together provide a suitable medium to minimize damage during freezing and thawing (Bustamante-González et al., 2019).
Currently, there is a specific protocol of sperm cryopreservation for the C. jordani species (Bustamante-González et al., 2021) where four cryoprotective agents (CPAs) effect was evaluated: dimethyl sulfoxide (DMSO), methanol (MeOH), ethylene glycol (EG) and glycerol (GL) in five concentrations: 2%, 6%, 10%, 14% and 16% v/v. Results showed that C. jordani sperm can be cryopreserved in MeOH 10% and EG14%, at 10 and 5 min equilibrium times whit 54.5 ± 1.0% and 53.1 ± 1.0 % post-thaw motility percentages, respectively, thawed at 40°C. This demonstrates the progress and interest in implementing and improving the proposed cryopreservation protocols for the genus Chirostoma.
Therefore, aim of present investigation was to evaluate fertilizing capacity in cryopreserved sperm of C. jordani, as a viable alternative for its conservation and/or assisted reproduction and the genus.
Materials and Methods
Present investigation was carried out under collection permission DGOPA. 07343.310810.4128 SEMARNAT, Mexico. The sample of organisms was reduced to the minimum necessary to cover the protocol.
It was collected 600 sexually mature males (semen presence, weight 3.9 ± 0.4 g, 8.9 ± 0.3 cm total length) and 150 sexually mature females (ova presence, 4.5 ± 0.6 g, 8.5 ± 0.4 cm total length). Fish was collected in Atlangatepec dam, Tlaxcala, Mexico.
Males were anesthetized with clove essence (0.01 mL/L water), before semen collection. Genital opening was cleaned with absorbent paper; semen was obtained by light pressure on abdominal and it was collected with a micropipette (10 µL) (Bustamante-González et al., 2018).
Sperm concentration was determinate individually (n = 100), from a stock solution (94 µL NaCl 9%, 5 µL formaldehyde 4% plus 1 µL semen) (Bustamante-González et al., 2018). Sperm number was estimated (cells per/µL) in Neubauer camera under microscope at 100X (Olympus Optical BX41TF®, Olympus, Tokyo, Japan).
Sperm motility was determinate in each of 100 males. It was active 1 µL fresh semen in 15 µL dam water and it was observed under optical microscope at 100X (Bustamante-González et al., 2021). This volume allowed sperm activation and motility estimation. Sperm motility was evaluated by one person to standardize bias in measurements and estimated from 0% to 100% subjectively (Borges et al., 2005; Bustamante-González et al., 2018).
Sperm cryopreservation was carried out according to the specific protocol of sperm cryopreservation for C. jordani proposed by Bustamante-González et al. (2021). This one consists of suspending 10 μL semen in an extender solution composed by 0.09 M sodium glutamate, 0.04 M fructose, 0.003 M magnesium acetate and 0.05 M potassium acetate (310 mOsmol/kg) (Lake & Ravie, 1984) in 1:1 ratio, in each of DMSO 10%, MeOH 10% and EG 14%, whit 10, 10 and 5 min equilibrium times, respectively, at 4°C. Later, samples are transferred to 0.25 mL straws placed 3 cm above liquid nitrogen for 5 min. Straws are stored in liquid nitrogen (–196°C) in a thermos (Cryosystem series XC 20/ 20, Custom Biogenic Systems, Bruce Township, MI, USA). After 15 days, straws are thawed by immersion individually, in water at 40°C for 15 s. Samples are transferred to 0.1 mL microtubes, they are homogenized and it is determined motility percentage (1 μL sample plus 5 μL dam water, as activator solution) by triplicate, under Olympus Optical BX41TF® microscope at 100X.
Sexually mature females (n = 150), oocytes present, were anesthetized with clove essence (0.1 mL/L water) before oocytes extraction. Oocytes was obtained by light pressure on abdominal. First 60 oocytes were obtained from each female and they were distributed in Petri boxes (60 × 15 mm) by quadruplicate.
Fertilizing capacity in fresh was done as follow: 1) Eight groups were conformed, each with four Petri boxes and each box with 60 oocytes, 2) Fresh semen from 10 males pooled (90% motility percentage), 3) each group was fertilized with following semen volumes: 0.4, 0.2, 0.1, 0.08, 0.06, 0.04, 0.02 and 0.01 µL; each semen volume was activated with 2.0, 1.0, 0.5, 0.4, 0.3, 0.2, 0.1 and 0.05 µL dam water (Table 1), 4) after semen was activated, it was aspirated with a micropipette and poured directly onto the oocytes, in each Petri boxes, 5) fertilization (%) was determined by perivitelline space formation, after 10 minutes.
Fertilizing capacity post-thaw was carried out as follows: 1) three groups were formed with four batches, each batch with four Petri boxes and each box with 60 oocytes, 2) 16 straws were post-thaw from each CPAs: DMSO, MeOH and EG, 3) motility percentage was verified (≥ 50%), 4) each batch was fertilized with different semen volume, in each group: batch 1: 10 µL, batch 2: 5 µL, batch 3: 2.5 µL and batch 4: 1.25 µL. Each semen volume was activated with 50, 25, 12.5 and 6.25 µL dam water (Table 2), 5) after semen was activated, it was aspirated with a micropipette and poured directly onto the oocytes, in each Petri boxes, 6) fertilization (%) was determined by perivitelline space formation, after 10 minutes.
Volume, sperm concentration and sperm motility were expressed as mean ± SD from fresh semen samples. Normal distribution data was verified by Shapiro-Wilk test (p < 0.05). One-way ANOVA was employed (95% confidence, α = 0.05), to determine cryopreservation effect over sperm motility. Concentrations and CPAs effects over sperm motility percentages were analyzed through a one-way ANOVA (95% confidence, α = 0.05) followed by Tukey test (α = 0.05). Therefore, fertility (%) between fresh and cryopreserved semen volumes was analyzed (ANOVA, p < 0.05). Data were analyzed by SigmaPlot-14 program (Systat Software, San Jose, CA, USA).
Results
Average semen volume of C. jordani was 3.86 ± 1.53 µL, with a sperm concentration 1.8 ± 0.3 × 106 µL–1 and ≥ 95% motility percentage.
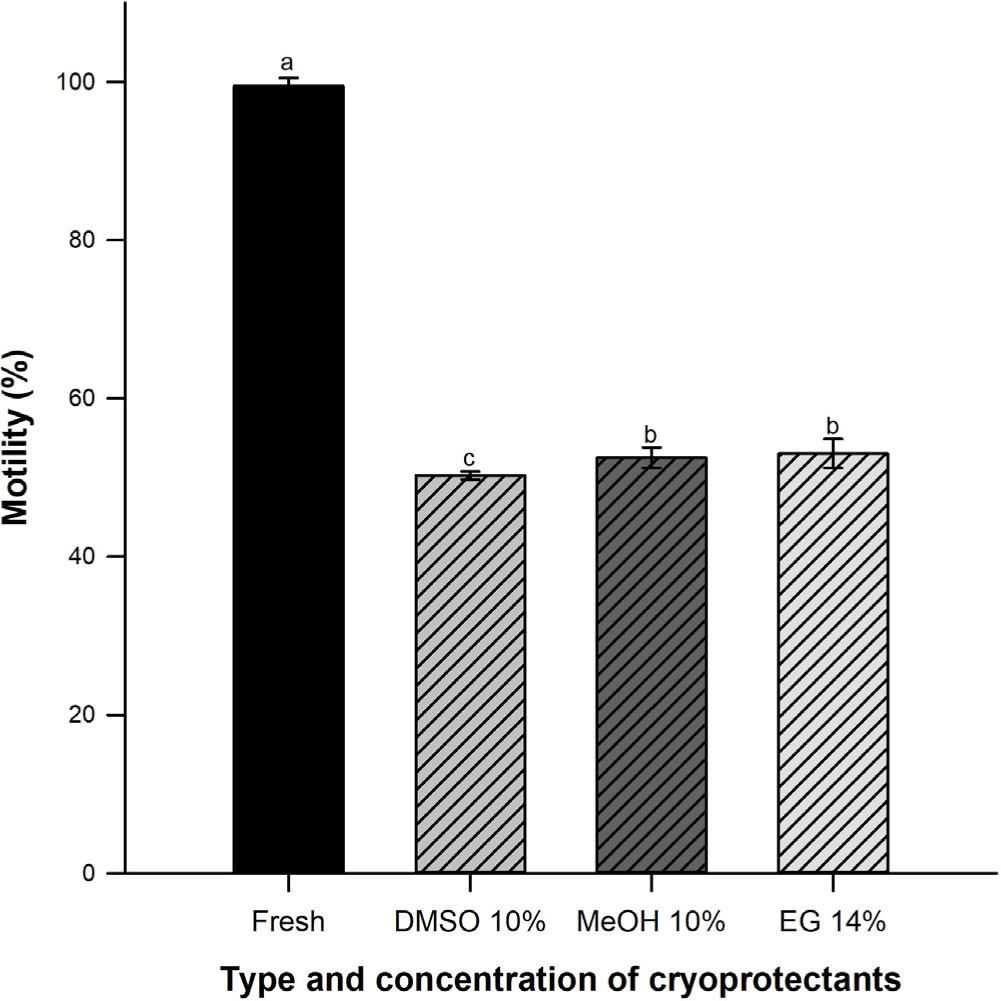
Sperm motility percentage in fresh samples decreased during cryopreservation process (p < 0.05). There were not differences among EG (53.5 ± 1.9%) and MeOH (53.3 ± 1.3%) (p > 0.05) but DMSO varied significantly (50.3 ± 0.5%) (Fig. 1).
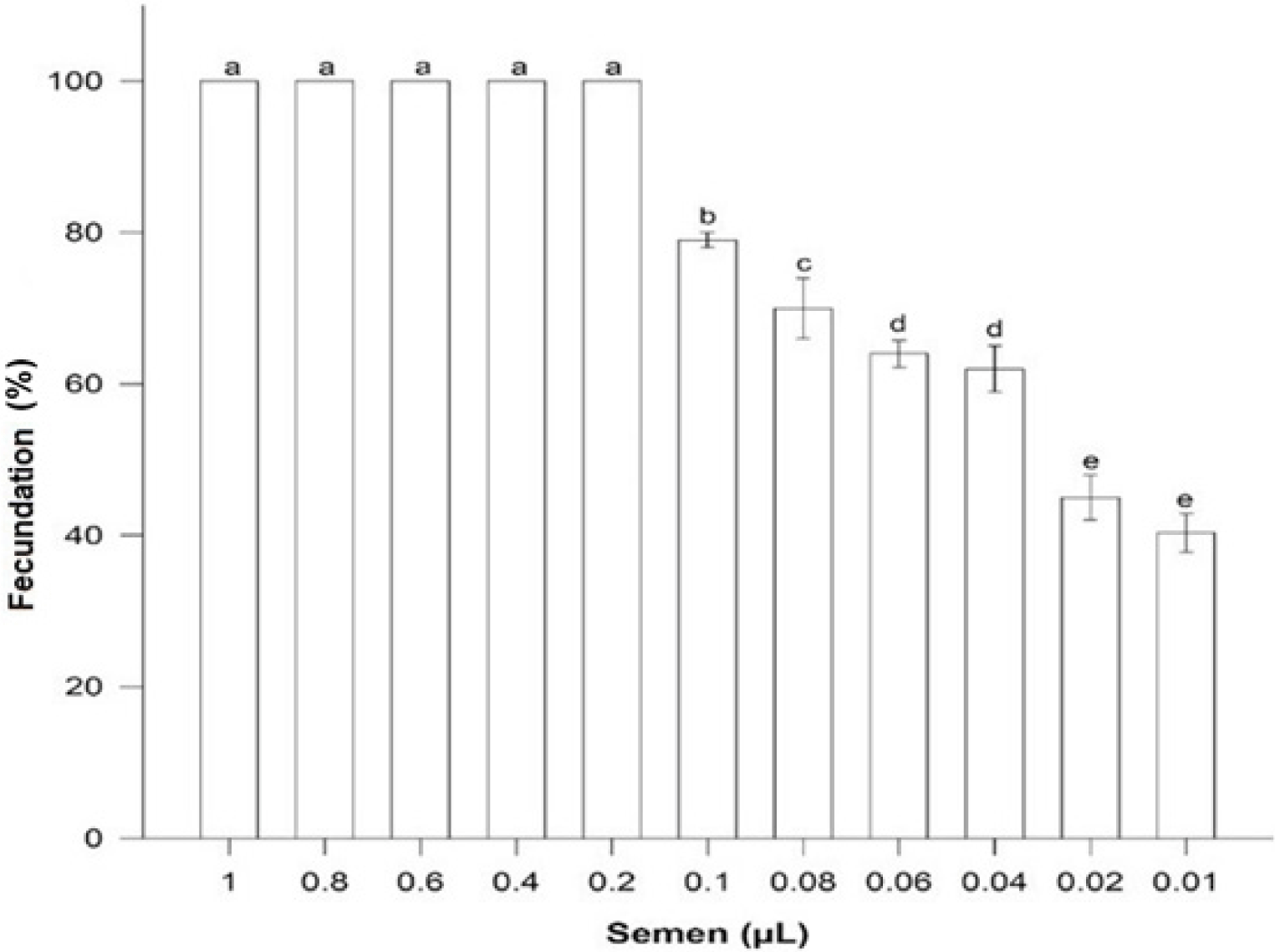
A volume of 0.2 µL fresh semen was enough to fertilize 100% oocytes (n = 60) with a relationship 6,000:1 sperm: oocyte. Volumes higher were not different (p > 0.05) but fertilization success was less with minor volumes (p < 0.05) (Fig. 2).
Semen cryopreserve volumes were greater to fertilize same number of oocytes (n = 60) (p < 0.05). It was needed 10 µL in samples cryopreserved in DMSO (150,900 sperm: oocyte (Fig. 3A), and 5 µL of MeOH and EG (79,950 and 80,250 sperm: oocyte, respectively) (Fig. 3B and 3C).
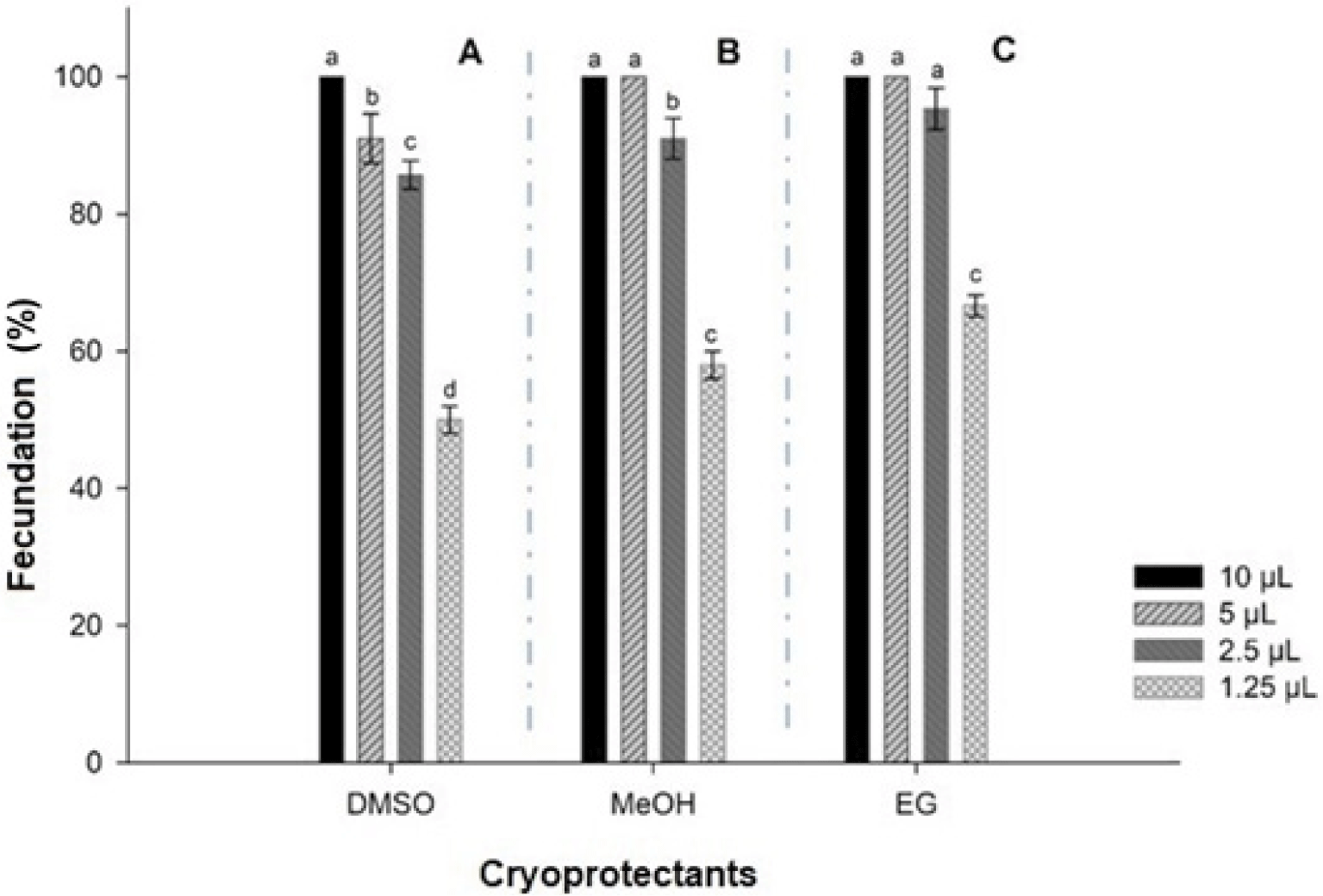
There was no significant difference (p > 0.05) among batches of oocytes in MeOH fertilized with 10 and 5 µL semen, but they were different from batches fertilized with 2.5 and 1.5 µL semen (p < 0.05) (Fig. 3B). Finally, samples oocytes cryopreserved in EG were not different fertilized with 10, 5 and 2.5 µL semen, but all of them were from batches fertilized with 1.25 µL semen (Fig. 3C).
Discussion
Cryopreservation had an effect over fertilizing capacity of semen, because their viability and motility decreased during thaw and post-thaw process, considerably. Because of this, it has been registered it is necessary a greater volume or concentration of semen to obtain percentages similar to those with fresh semen (Bozkurt & Yavaş, 2017; Ciereszko et al., 2014; Di lorio et al., 2019; Du et al., 2018; Kommisrud et al., 2020).
In all cryopreservation protocol is a lost in motility percentage, due to sensitivity degree of sperm to CPAs toxicity, osmotic stress of extender solution over cell and oxidative stress of cryopreservation effect (Judycka et al., 2020; Nynca et al., 2019).
Osmotic stress is attributed to permeable CPAs (DMSO, MeOH, EG, GL, propanediol) are easily spread in cell (Gironi et al., 2020). Their principal function in cryopreservation is to prevent ice crystals formation inside cell. However, the CPAs can have next behavior during the process: 1) Ice is formed in extracellular space during cryopreservation. This gives rise to increase solutes concentration and causes an osmotic imbalance between inside and outside of cell. 2) Cooling rate should be slow enough to allow solutes and water are exchanged, in order to keep minimum imbalance osmotic. 3) When icy melts, extracellular space is hypotonic relative to inside cell, hence it can cause rapid ingress of water and give rise to cell lysis (Notman et al., 2006). Therefore, it is necessary to standardize equilibrium time according CPAs which depends on CPAs concentration and target species.
Besides, Gironi et al. (2020) and Kumari & Kashap (2019) report CPAs, in low concentrations, induces disorder of fatty acid tails of membrane phospholipids, in addition to increase phospholipid bilayer fluidity; high concentrations of these cause pores formation through membrane and their later increases cause plasma membrane disintegration. These results could support why sperm motility percentage depends of concentration and type CPAs. A previous study showed low concentrations of these agents induced lower motility percentage and there was a maximum motility value in higher CPAs, and from which it was observed a percentage decrease (Bustamante-González et al., 2021). This behavior may be due to high concentrations agents which form transient pores water or membrane disintegration, in extreme cases, both affect sperm viability (Gironi et al., 2020; Kumary & Kashap, 2019).
In addition, cryopreservation generates numerous structural and physiological changes to spermatozoa: thin membrane, increased fluidity, transient water pores through phospholipid bilayer, phospholipid bilayer breakdown, plasma membrane rupture, altered mitochondrial potential, reactive oxygen species production, DNA fragmentation, protein function, adenosine triphosphate (ATP) concentrations alterations, intracellular calcium homeostasis, among others, together affect fertilization (Ahn et al., 2018; Bustamante-González et al., 2019; Díaz et al., 2019; Figueroa et al., 2019; Gironi et al., 2020; Kumari & Kashyap, 2019; Lee-Estevez et al., 2019; Malcervelli et al., 2020; Schrader et al., 2016). Because of this, it is estimated 30% sperm motility loss for fertilization (Valipour, 2021). It was presented a loss about 50% in this study, similar to previous research (Bustamante-González et al., 2021); this motility loss may be due to cryopreservation protocol causes these structural and physiological changes to spermatozoa. These changes have been evaluated in other fish species (Cabrita et al., 2014; Díaz et al., 2019; Figueroa et al., 2019; Sandoval-Vargas et al., 2020).
Success of a protocol cryopreservation is keeping sperm motility to fertilization. several researches have reported fertilizing capacity of sperm cryopreserved, mainly in model species of Salmonidae and Cyprinidae, where fertilizing capacity sperm decreases significantly during cryopreservation process, regarding oocytes fertilized with fresh semen.
Nevertheless, these researches have used same sperm concentration per oocyte, in fresh as cryopreserved, without considering that motility percentage, viability and ATP decrease with cryopreservation, significantly (Figueroa et al., 2019; Kommisrud et al., 2020; Sandoval-Vargas et al., 2020). For this reason, some authors recommend using excessive amounts of cryopreserved semen to increase fecundation (Tekin et al., 2007). Similar results were obtained in present study.
Sperm-oocyte ratio depends from species, mainly on assisted reproduction, since a greater number of oocytes can be fertilized with a lower sperm concentration. An advantage from assisted reproduction is to limit sperm displacement space so they invest their energy in penetrating the micropyle. This could explain differences in sperm number needed to fertilization among species, which is between 15 × 106 and 5 × 103 fresh sperm per oocyte, with a fecundation higher 90%. Similar results were obtained with fresh semen from C. jordani.
Species habitat is another important variable to consider. Different fertilization (%) have been obtained in brown trout populations that inhabit different rivers within same basin. This answer may explain sperm resistance to type and concentration of extender solution and CPAs (Ciereszko et al., 2014; Nynca et al., 2019), since both affect sperm motility. Results obtained in present research show that sperm concentration per oocyte depends on cryopreservation protocol, fertilization, species and even, among individuals of the same species, and they coincide with those obtained by other studies (Bozkurt & Yavaş, 2017; Ciereszko et al., 2014; Di lorio et al., 2019; Du et al., 2018; Kommisrud et al., 2020).







